From Wikipedia, the free encyclopedia
Pharmaceutical compound
2C-G , or 2C-G-0 , also known as 3,4-dimethyl-2,5-dimethoxyphenethylamine or as 3-methyl-2C-D , is a psychedelic phenethylamine of the 2C family.[ 1] synthesized by Alexander Shulgin , it has structural and pharmacodynamic properties similar to 2C-D and Ganesha (G).[ 1] homologues , which are known as the 2C-G series of compounds.[ 1]
In Alexander Shulgin 's book PiHKAL (Phenethylamines I Have Known and Loved ), the dose range is listed as 20 to 35 mg orally .[ 1] Ganesha , and are extremely long lasting; the duration is 18 to 30 hours.[ 1] Visual effects are muted or absent, and it is described as an "insight-enhancer".[ 1] 2C series, 2C-G is nearly as potent as its amphetamine form.[ 1]
The chemical synthesis of 2C-G has been described.[ 1]
Several homologues of 2C-G were also synthesized by Alexander Shulgin .[ 1] 2C-G-3 , 2C-G-5 , and 2C-G-N .[ 1] 2C-G-4 , and 2C-G-6, are possible to synthesize in principle but impossible or extraordinarily difficult to do so in practice.[ 1]
Compound
Details
Structure
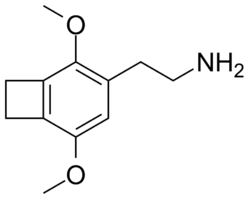
2C-G-1
CAS : 2888537-47-9 The synthesis of this compound has not been reported, but it is described prophetically in WO2022271982
2C-G-2
CAS : 2888537-48-0 The synthesis of this compound has not been reported, but it is described prophetically in WO2022271982
2C-G-3
CAS : 207740-19-0 Dose : 16–25 mg Duration : 12–24 hours Effects : Some visual effects. General euphoria with an underlying sense of paranoia.
2C-G-4
CAS : 952006-59-6 Partially synthesized but not tested.
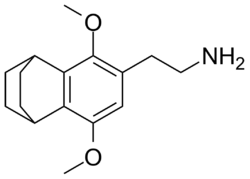
2C-G-5
CAS : 207740-20-3 Dose : 10–16 mg Duration : 32–48 hours Effects : Similar to 2C-B for some users. General euphoria (sometimes followed by irritability), often leading to tiredness (likely due to duration).
2C-G-6
CAS : 2888537-49-1 The synthesis of this compound has not been reported, but it is described prophetically in WO2022271982
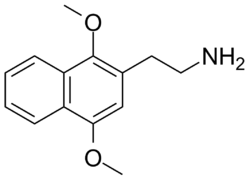
2C-G-N
CAS : 207740-21-4 Dose : 20–40 mg Duration : 20–30 hours Effects : Stimulation similar to that caused by amphetamines. General sense of unease or unfriendliness for most. 2C-G-N is sometimes called 2C-NPH due to the naphthalene portion of the molecule.
2C-G was first described in the literature by Alexander Shulgin in his 1991 book PiHKAL Phenethylamines I Have Known and Loved ).[ 1]
Society and culture [ edit ] As of October 31, 2016; 2C-G is a controlled substance (Schedule III) in Canada.[ 2]
2C-G and all other compounds featuring in PiHKAL are Class A drugs in the United Kingdom .
In the United States 2C-G is considered a Schedule I controlled substance as a positional isomer of 2C-E and DOM .[ 3]
No ring subs. 4-Hydroxytryptamines 5-Hydroxytryptamines 5-Methoxytryptamines Other ring subs.
2,N ,N -TMT 4,N ,N -TMT 5-Bromo-DMT 5-Chloro-DMT 5-Fluoro-DMT 5-N ,N -TMT 7,N ,N -TMT 5-MeO-2,N ,N -TMT 5-MeO-4,N ,N -TMT 6-Fluoro-DMT 6-Hydroxy-DET (6-HO-DET) Bretisilocin (GM-2505; 5-fluoro-MET) α-Alkyltryptamines Others
Ergolines /lysergamides (e.g., LSD )β-Carbolines and Harmala alkaloidsharmine , harmaline , 6-methoxyharmalan )Iboga alkaloids18-MAC , 18-MC , coronaridine , ibogaine , ibogamine , ME-18-MC , noribogaine , tabernanthine , voacangine )Ibogalogs (e.g., ibogainalog )O -MethylnordehydrobufoteninePartial ergolines (e.g., NDTDI , RU-28306 , CT-5252 )Piperidinylethylindoles (e.g., pip-T )Pyrrolidinylethylindoles (e.g., pyr-T , 5-MeO-pyr-T )Pyrrolidinylmethylindoles (e.g., MPMI , 4-HO-MPMI (lucigenol) , 5-MeO-MPMI )Tetrahydropyridinylindoles (e.g., RU-28253 (5-MeO-THPI) , NEtPhOH-THPI )
Benzofurans (e.g., 5-MeO-DiBF , dimemebfe (5-MeO-BFE) , mebfap )Benzothiophenes (e.g., 3-APBT )Indazolethylamines (e.g., AL-38022A , O -methyl-AL-34662Indenylethylamines (e.g., C-DMT )Isotryptamines (e.g., 6-MeO-isoDMT , Ro60-0175 )MYCO-005 Quinolinylethylamines (e.g., mefloquine )
Others: 2C-B-AN 2C-DB 2C-G-x (e.g., 2C-G-3 , 2C-G-5 )β-Keto-2C-B (βk-2C-B) β-Keto-2C-I (βk-2C-I) β-Methyl-2C-B (BMB) BOB , BOD , BOH-2C-B )HOT-2 , HOT-7 , HOT-17 )N -Ethyl-2C-B2CD-2-ETO , 2CD-5-ETO , 2CE-5-ETO , 2CE-5iPrO , 2CT2-5-ETO , ASR-2001 (2CB-5PrO) ) Others
2-TOET 2-TOM 25B-NAcPip 4-HA 5-TOET 5-TOM Benzofurans (e.g., 5-APB , 5-APDB , 6-APB , 6-APDB , F , F-2 , F-22 )Benzothiophenes (e.g., 5-APBT , 6-APBT )CT-5172 DMAs (e.g., 2,4-DMA , 3,4-DMA )Fenfluramine MMA (3-MeO-4-MA) Norfenfluramine 25D-NM-NDEAOP , DOB-NDEPA , DOI-NDEPA , DOM-NDEPA , DOTFM-NDEPA , M-NDEPA , TMA-2-NDEPA )PMA (4-MA) TMA-3 , TMA-4 , TMA-5 )TOMSO ZDCM-04
1-Aminomethylindanes (e.g., 2CB-Ind , jimscaline )2-Aminoindanes (e.g., DOM-AI )3-Benzazepines (e.g., lorcaserin )3-Phenylpiperidines (e.g., LPH-5 , LPH-48 )Benzocyclobutenes (e.g., 2CBCB-NBOMe , TCB-2 , tomscaline )Benzoxepins (e.g., BBOX , IBOX , TFMBOX )DMBMPP (juncosamine) Ergolines /lysergamides (e.g., LSD )Glaucine Partial ergolines (e.g., NDTDI , DEIMDHPCA , DEMPDHPCA , DEMTMPDHPCA , DEMNDHPCA )Phenylcyclopropylamines (e.g., DMCPA , TMT )Phenyloxazolamines (aminorexes ) (e.g., 2C-B-aminorex )Pyridopyrroloquinoxalines (e.g., IHCH-7113 )Z3517967757 ZC-B
Others
Arylpiperazines (e.g., 2C-B-PP , 2-NP , mCPP , MK-212 , ORG-12962 , pCPP , pFPP , quipazine , TFMPP )Dihydrobenzoxazines (e.g., efavirenz )Phenoxyethylamines (e.g., CT-4719 , ORG-37684 )Pyridopyrroloquinoxalines (e.g., IHCH-7113 )Quinazolinylethylamines (e.g., RH-34 ) Natural sources
Tryptamines: Acacia spp.Acacia acuminata Acacia confusa Ayahuasca and vinho de Jurema (e.g., Psychotria viridis (chacruna)Dipolopterys cabrerana (chaliponga, chacruna)Mimosa tenuiflora (Mimosa hostilis ; jurema)Brosimum Brosimum acutifolium (takini)Hallucinogenic snuffs (e.g., Anadenanthera peregrina (yopo, jopo, cohoba, parica, ebene)Anadenanthera colubrina (vilca, cebil)Incilius alvarius (Bufo alvarius ; Colorado River toad, Sonoran Desert toad; bufo)Psilocybin-containing mushrooms (magic mushrooms, shrooms) (e.g., Psilocybe cubensis Psilocybe mexicana (teonanacatl)Lysergamides: Achnatherum robustum (sleepy grass)Epichloë spp.Ergot (Claviceps ) (e.g., Claviceps purpurea Claviceps paspali Morning glory (Convolvulaceae) seeds (e.g., Ipomoea tricolor (tlitliltzin, badoh negro; Ipomoea violacea )Ipomoea corymbosa (coaxihuitl, ololiúqui; Rivea Corymbosa , Turbina Corymbosa )Argyreia nervosa (Hawaiian baby woodrose; HBWR)Periglandula spp.Periglandula ipomoeae Periglandula clandestina
Phenethylamines Amphetamines Phentermines Cathinones Phenylisobutylamines (and further-extended) Catecholamines (and close relatives) Cyclized
Phenylalkylpyrrolidines 2-Benzylpiperidines (phenidates ) Phenylmorpholines (phenmetrazines) Phenyloxazolamines (aminorexes) Isoquinolines andtetrahydroisoquinolines 2-Aminoindanes 2-Aminotetralins Others / unsorted
1-Aminomethylindanes (e.g., 2CB-Ind , AMMI , bromojimscaline , jimscaline )2-ADN 2-Benzhydrylpyrrolidine 2C-B-5-hemiFLY-α6 (BNAP) 2C-B-PYR 2CBecca 2CB7 2CJP 2CLisaB 2CLisaH 3-Benzazepines (e.g., fenoldopam , lorcaserin , 7-chlorolorcaserin , SCHEMBL5334361 )3-Benzhydrylmorpholine 3-Phenylpiperidines (e.g., 3-phenylpiperidine , 3-PPP , OSU-6162 (PNU-96391) , LPH-5 , LPH-48 , Z3517967757 (Z7757) )6-AB AL-1095 Aminochromes (e.g., adrenochrome , adrenolutin )Benzocyclobutenes (e.g., 2CBCB-NBOMe , bromotomscaline , S33005 , TCB-2 , tomscaline )Benzoxepins (e.g., BBOX , IBOX , TFMBOX )Butyltolylquinuclidine Camfetamine Cypenamine (trans -2-phenylcyclopentylamine) Diphenidine Diphenylprolinol DMBMPP Ergolines (e.g., LSD )Fencamfamin GYKI-52895 HDMP-29 Ivabradine Methoxphenidine Methylmorphenate Milnacipran MT-45 2-Naphthylamine Org 6582 Partial ergolines (e.g., NDTDI , RU-27849 , DEIMDHPCA , DEMPDHPCA , DEMPDHPCA-2C-D , RU-27251 )PF-592,379 Phenylcyclopropylamines (e.g., DMCPA , TMT , tranylcypromine )Phenylpiracetams (e.g., phenylpiracetam , MRZ-9547 , RGPU-95 )Pyridopyrroloquinoxalines (e.g., lumateperone , deulumateperone , IHCH-7079 , IHCH-7086 , IHCH-7113 , ITI-1549 )Tetrahydrobenzopyranylamines (e.g., CT-5126 )Tolazoline Tricyclics (e.g., AMDA , AMDH , benzoctamine , dizocilpine , SpAMDA )ZC-B
Related compounds
2-Furylethylamine 2-Pyrrolylethylamine 3-Pyrrolylethylamine 3-Pyrrolylpropylamine 2-Tetrahydrofurylethylamine 4-Benzylpiperidine 7-AB Alkylamines (e.g., 1,3-DMBA Tooltip 1,3-dimethylbutylamine , 1,4-DMAA Tooltip 1,4-dimethylamylamine , heptaminol , iproheptine , isometheptene , methylhexanamine/1,3-DMAA , octodrine , oenethyl , tuaminoheptane )Benzylamines (e.g., benzylamine , α-methylbenzylamine , MDM1EA , ALPHA , M-ALPHA , pargyline )Benzylpiperazines (e.g., benzylpiperazine , MDBZP , fipexide )Cyclohexylaminopropanes (e.g., propylhexedrine , norpropylhexedrine )Cyclopentylaminopropanes (e.g., isocyclamine , cyclopentamine )Phenoxyethylamines (e.g., 3,4,5-trimethoxyphenoxyethylamine , CT-4719 , ORG-37684 )Phenylalkenylamines (e.g., phenylbutenamine )Phenylalkynylamines (e.g., phenylbutynamine )Phenylpiperazines (e.g., 1-phenylpiperazine , mCPP Tooltip meta-chlorophenylpiperazine , TFMPP Tooltip trifluoromethylphenylpiperazine , oMPP Tooltip ortho-methylphenylpiperazine , pFPP Tooltip para-fluorophenylpiperazine , pMeOPP Tooltip para-methoxyphenylpiperazine )Phenylpropylamines (e.g., phenylpropylamine , homo-MDA , homo-MDMA )Thienylaminopropanes (thiopropamines) (e.g., thiopropamine , methiopropamine , thiothinone )