Potassium peroxymonosulfate
This article has multiple issues. Please help improve it or discuss these issues on the talk page. (Learn how and when to remove these messages)
|
 | |
| Names | |
|---|---|
| IUPAC name
Potassium peroxysulfate
| |
| Other names | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.030.158 |
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
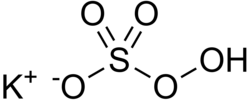
| KHSO5 | |
| Molar mass | 152.2 g/mol (614.76 g/mol as triple salt) |
| Appearance | Off-white powder[dubious – discuss][citation needed] |
| Decomposes[dubious – discuss][citation needed] | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Oxidant, corrosive |
| NFPA 704 (fire diamond) | |
| Safety data sheet (SDS) | ChemicalBook.com SDS[4] |
| Related compounds | |
Related compounds
|
Potassium persulfate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Potassium peroxymonosulfate, also referred to as potassium peroxysulfate and potassium monopersulfate (KMPS), formula KHSO5, is a chemical compound that is the mono-potassium salt of peroxymonosulfuric acid (Caro's acid)[2] In common applications, it is often referred to more simply as monopersulfate, MPS,[3] or by one of its commercial names—as it is a constituent of widely used oxidizing agents, for instance, the triple salt 2KHSO5·KHSO4·K2SO4 known especially by its early trade name, Oxone® (from DuPont de Nemours).[5] In such formulations, it functions under a variety of conditions, including homogeneous solutions and biphasic mixtures, as a stoichiometric oxidizing agent.[1] Underlying that use is its standard electrode potential, which for potassium peroxymonosulfate, per se, is +1.81 V (half reaction generating hydrogen sulfate, HSO4–). Applications of agents that include the MPS compound extend, for example, to uses in laboratory and industrial oxidative chemical conversions,[1] and in practical cleaning, whitening, and disinfection contexts.[6][better source needed][3]
Synonyms
[edit]The title compound is referred to by the IUPAC as potassium peroxysulfate, and more generally, as well, by a long series of more and less common name variants that alter the order of the name components (potassium, the peroxy-function, and the sulfuryl-core), sometimes altering the "peroxy-" to "peroxo-", and sometimes inserting "hydrogen" or "mono-" as a prefix preceding the sulfuryl component.[2] Frequently seen names include the IUPAC and title names, as well as potassium monopersulfate (KMPS) and its shortened variant, monopersulfate (MPS),[2][3] potassium hydrogen persulfate, and names derived from it's precursor, Caro's acid (potassium caroate and Caro's acid potassium salt).[1][2] The title compound is sometimes stated as being informally synonymous with commercial preparations containing it, e.g., Oxone® and Caroat®, see following.[1] For further explicit variations, see the CAS Common Chemistry entry for the title compound.[2]
Properties
[edit]This section needs expansion with: a standard introductory presentation of the various chemical and physical properties of the title compound. You can help by adding to it. (November 2025) |
This section relies largely or entirely on a single source. (November 2025) |
Chemical properties
[edit]Potassium peroxymonosulfate, KHSO5, is the mono-potassium salt of peroxymonosulfuric acid (Caro's acid).[2][1] Its discussion often centers on its formulations as a triple salt 2KHSO5·KHSO4·K2SO4, which is sold under registered trademarks, e.g., Oxone®, Curox®, and Caroat®.[1] Description of chemical and physical properties must make clear whether one is describing research aspects of the pure substance,[citation needed] versus the aspects of the widely used, commercially available formulations.[1]
Purity of preparations and reagents containing the title compound can be determined by iodometric titration.[1] A practical encyclopedia or organic reagents has indicated that the presence of heavy metal salts catalyzes the decomposition of the title compound in its triple salt formulation.[1]
Electrochemistry
[edit]The standard electrode potential for potassium peroxymonosulfate is +1.81 V with a half reaction generating the hydrogen sulfate (pH = 0):[7][non-primary source needed]
- HSO−5 + 2H+ + 2e− → HSO−4 + H2O .
Physical properties
[edit]The formulation of the title compound as the triple salt, 2KHSO5·KHSO4·K2SO4, was created to provide a form of KHSO5 that is "convenient to handle and store"; in this formulation, it is a "white, granular, free flowing solid", its density is reported as 1.12–1.20 grams per cubic centimeter, and it is reported to decompose on melting point measurement.[1] The same formulation is described as being soluble in water (25.6 grams per 100 grams water at 20◦C), as well as in aqueous methanol, ethanol, and acetic acid, but as being insoluble in "common organic solvents".[1] It can, however, be applied in biphasic systems, owing to its water solubility, through use of "an immiscible cosolvent and a phase-transfer catalyst".[1]
Toxicity
[edit]Formulated as its triple salt, the title compound and its co-formulants have low toxicity, but nevertheless require adequate ventilation (and standard chemical handling precautions[citation needed]) for its use, to reduce exposure to its dust, which is "irritating to the eyes, skin, nose, and throat".[1]
Triple salt formulations (Oxone®, etc.)
[edit]The potassium peroxymonosulfate-containing formulation, the triple salt 2KHSO5·KHSO4·K2SO4, has a molecular weight of 614.7, with KHSO5 composing, in 2008 (during its manufacture by DuPont, see following), an estimated 43-45% of it, by weight, of which 5.2% active oxygen was theoretically possible, and 4.7% was typically observed.[5] (In 2012, a review was reporting the KHSO5 estimate to be "about 50% per mole" of triple salt.[1]) The stability advantage notwithstanding (see following), methods were developed to deliver a forms of the title compound that required smaller amounts in reactions, and this was achieved on large scale in 2002 via preparations of purified KHSO5·H2O.[1][8][needs update]
The triple salt formulations have a longer shelf life than potassium peroxymonosulfate, per se.[1][5][better source needed] Available in commercial formulations, originally from DuPont deNemours Chemical (early trade name, Oxone®),[5][better source needed] now from Lanxess Deutschland Gmbh (as Oxone™),[6] it has also been available from other manufacturers under other trade names (e.g., as Curox® and Caroat®).[1] These products are of wide commercial value.[citation needed]
The triple salt products are produced from peroxysulfuric acid,[citation needed] which is generated in situ by combining fuming sulfuric acid (oleum) and hydrogen peroxide.[citation needed] Careful neutralization of this solution with potassium hydroxide allows the crystallization of the triple salt.[citation needed]
Uses
[edit]Cleaning
[edit]This section needs expansion with: more authoritative and up-to-date coverage of the uses, which extend to water treatment, pulp and paper, disinfection, and odor control (see Laxness, op. cit.). You can help by adding to it. (November 2025) |
Oxone®-type products is used widely for oxidative processes that result in decomposition of organic contaminants, and therefore in cleaning, whitening, and disinfection.[6][better source needed] For instance, it can be used to whiten materials used in dental health practices,[9][6] to clean materials in the manufacture of microelectronics,[9][10][11][6] and sees extensive use as an oxidizer to decompose organic contaminants in swimming and other recreational water pools.[6][better source needed][3] Use of formulations containing the title compound in pool water quality management can interfere with determinations of chlorination assay, using a standard ferrous ammonium sulfate, N,N′-diethyl-p-phenylenediamine (FAS-DPD) method, if added reagents and steps are not followed to neutralise the KMPS (potassium monopersulfate / peroxymonosulfate).[12][better source needed][13][better source needed]
Preparative chemistry
[edit]This section needs expansion with: a better focused discussion on the most important reactions, and an update with latest broadly used synthetic methods, including inorganic (per DuPont, metal ion, and halogen and heteroatom oxidations), and expanded/refined organic examples; see Shi-Burke and Buckley updates to Crandall, and other, broader sources. You can help by adding to it. (November 2025) |
Potassium peroxymonosulfate and Oxone®-type products are versatile oxidants in organic chemistry and other synthetic arenas.[1][14][5] Oxone®-type products oxidize terminal alkenes to epoxides, and cleave internal alkenes, providing two equivalents of carboxylic acid.[citation needed] They can be used to convert aldehydes to carboxylic acids; in the presence of alcoholic solvents, the corresponding esters may be obtained.[15][non-primary source needed]
Oxone®-type products convert ketones to dioxiranes, which can be used for diverse oxidations in organic synthesis.[citation needed] The reagent dimethyldioxirane (DMDO) forms upon treatment of acetone with an Oxone®-type product; dioxiranes are versatile oxidants, especially in the epoxidation of olefins,[16] and in the oxidation of other unsaturated functionalities, heteroatoms, and even some alkane C-H bonds.[17]

Oxone®-type products are used in the production of some organic periodinanes, notably the oxidation of 2-iodobenzoic acid to 2-iodoxybenzoic acid (IBX).[18][non-primary source needed]

Peroxymonosulfate-driven conversions can be used with sulfides and selenides to prepare sulfones and selenones, with anilines and amino sugars to provide nitro compunds, oximes to provide nitro compunds (in aqueous buffered conditions) or to return the parent carbonyl compounds (in the presence of alumina, with microwave heating), primary and secondary amines to provide hydroxylamines (using adsorbed Oxone®) or N-nitrosation products (in the presence of sodium nitrite), pyridines and tertiary amines to provide amine oxides, and phosphorus(III) compounds to provide phosphono-compounds largely retaining configuration at phosphorus (with comparable outcomes when a sulfur or selenium atom replaces the phosphorus(III) lone pair).[1]
Examples of preparative scale oxidatives of these types are the conversion of an acridine derivative to the corresponding acridine-N-oxide,[19] and the synthesis of fluoromethyl phenyl sulfone, a reagent used in the synthesis of fluoroalkenes.[20]


The drawback of peroxymonosulfate anion insolubility in organic solvents (and thus proscription to aqueous, alcoholic, and phase transfer reaction conditions) has been addressed through the preparation of organic-soluble salts of Caro's acid, to replace the potassium of the title compound and Oxone®-type products; several have been prepared—e.g., the tetra-n-butylammonium, tetraphenylphosphonium, and benzyltriphenylphosphonium salts (nBu4NHSO5, Ph4PHSO5, and BnPh3PHSO5)—and they have been studied and found comparable in reactivity "in many cases" to Oxone®-type products.[1]
Other preparative chemical applications
[edit]In addition to the title compound, ammonium and sodium salts of HSO−5 are used in the plastics industry as radical initiators for polymerization.[citation needed] The title compound or its formulations have been used for decolorizing and deodorizing oils,[citation needed] as etchants,[clarification needed][citation needed] and as oxidative desizing agents for textile fabrics.[citation needed] It has also been investigated for use in processes aimed at delignification of wood.[21]
Further reading
[edit]- Crandall, Jack K.; Shi, Yian; Burke, Christopher P.; Buckley, Benjamin R. (September 14, 2012) [2001]. Paquette, Leo A. (ed.). Encyclopedia of Reagents for Organic Synthesis. New York, NY: Wiley & Sons. doi:10.1002/047084289x.rp246.pub3. ISBN 9780470842898. Retrieved November 3, 2025. The original 1995 print publication of this chapter by Crandall was vol. 3, on pages 295ff.
- DuPont Staff (2008). "DuPont™ Oxone® Monopersulfate Compound / General Technical Attributes" (PDF). du Pont de Nemours. Wilmington, DE: E.I. du Pont de Nemours & Co. Retrieved November 3, 2025 – via Ataman Kimya A.Ş.(AtamanKimya.com).
- Jakob, Harald; Leininger, Stefan; Lehmann, Thomas; Jacobi, Sylvia & Gutewort, Sven (July 15, 2007). "Peroxo Compounds, Inorganic". In Ley, Claudia (ed.). Ullmann's Encyclopedia of Industrial Chemistry. Weinheim, Germany: Wiley-VCH. doi:10.1002/14356007.a19_177.pub2. Retrieved November 3, 2025.
{{cite encyclopedia}}: CS1 maint: multiple names: authors list (link)
- Mundy, B.P.; Ellerd, M.G. & Favaloro Jr., F.G. (2005). "Oxone®". Name Reactions and Reagents in Organic Synthesis (2nd ed.). Hoboken, NJ: John Wiley & Sons. p. 828. ISBN 9780471739869. Retrieved November 3, 2025.
{{cite book}}: CS1 maint: multiple names: authors list (link)
- Page, P.C.B.; Barros, D.; Buckley, B.R.; Ardakani, A. & Marples, B.A. (2004). "Organocatalysis of Asymmetric Epoxidation Mediated by Iminium Salts under Nonaqueous Conditions". J. Org. Chem. 69 (10): 3595–3597. doi:10.1021/jo035820j. Retrieved November 3, 2025.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Presents an organic-soluble form of Oxone®, tetraphenylphosphonium peroxymonosulfate (Ph4PHSO5), and its successful deployment in the asymmetric epoxidation using peroxymonosulfate-generated oxaziridinium salts.
- Travis, B. R.; Ciaramitaro, B. P. & Borhan, B. (September 30, 2002). "Preparation of Purified KHSO5·H2O and nBu4NHSO5 from Oxone by Simple and Efficient Methods". Eur. J. Org. Chem.: 3429–3434. doi:10.1002/1099-0690(200210)2002:20<3429::AID-EJOC3429>3.0.CO;2-D. Retrieved November 3, 2025.
{{cite journal}}: CS1 maint: multiple names: authors list (link) The article has been made available by an author, at this link.
References
[edit]- ^ a b c d e f g h i j k l m n o p q r s t u v Crandall, Jack K.; Shi, Yian; Burke, Christopher P.; Buckley, Benjamin R. (September 14, 2012) [2001]. Paquette, Leo A. (ed.). Encyclopedia of Reagents for Organic Synthesis. New York, NY: Wiley & Sons. doi:10.1002/047084289x.rp246.pub3. ISBN 9780470842898. Retrieved November 3, 2025. This encyclopedia article has received two updates after the initial chapter by Crandall, by Yian Shi and Christopher P. Burke (first), and Benjamin R. Buckley (second), both of which appear in the September 2012 release of the online work.
- ^ a b c d e f g h CAS Staff (November 3, 2025). "Potassium peroxymonosulfate". CAS Common Chemistry (CommonChemistry.CAS.org). Columbus, OH: Chemical Abstracts Service (CAS), American Chemical Society. Retrieved November 3, 2025.
Other Names for this Substance: Peroxymonosulfuric acid, potassium salt (1:1) / Potassium peroxymonosulfate (KHSO5) / Peroxymonosulfuric acid, monopotassium salt / Monopotassium persulfate / Potassium monopersulfate // Potassium peroxomonosulfate... / Potassium hydrogenperoxymonosulfate / Caro's acid potassium salt / Potassium hydrogen persulfate / ... .
CAS RN: 10058-23-8. Licensed under the Attribution-Noncommercial 4.0 International License (CC BY-NC 4.0). - ^ a b c d e PHTA Recreational Water Quality Committee (June 11, 2019). "Tech Notes: Potassium Monopersulfate". Aqua Magazine (AquaMagazine.com). Madison, WI: AB Media Inc. Retrieved November 3, 2025.
Potassium Monopersulfate (monopersulfate, KMPS or MPS) is a white, granular, free-flowing peroxygen that provides powerful non-chlorine oxidation for a wide variety of uses. It is the active ingredient in most nonchlorine oxidizers used for pool and spa/hot tub oxidation. / Most non-chlorine oxidizers contain 45% of the active ingredient potassium monopersulfate, but blended compositions are also commercially available...
- ^ a b ChemicalBook.com Staff (July 5, 2025). "Chemical Safety Data Sheet MSDS / SDS: potassium hydrogenperoxomonosulphate... CAS: 10058-23-8". ChemicalBook.com. Retrieved November 3, 2025.
- ^ a b c d e DuPont Staff (2008). "DuPont™ Oxone® Monopersulfate Compound / General Technical Attributes" (PDF). du Pont de Nemours. Wilmington, DE: E.I. du Pont de Nemours & Co. Retrieved November 3, 2025 – via Ataman Kimya A.Ş.(AtamanKimya.com).
The active ingredient of Oxone® is potassium peroxymonosulfate, KHSO5 (CAS 10058-23-8), commonly known as potassium monopersulfate, which is present as a component of a triple salt with the formula 2KHSO5·KHSO4·K2SO4 potassium hydrogen peroxymonosulfate sulfate (5:3:2:2), [CAS 70693-62-8]).
[better source needed] - ^ a b c d e f Laxness Staff (2025). "Products and Brands / Brands / Oxone™". Laxness.com. Cologne, Germany: LanXess AG. Retrieved November 3, 2025.
Oxone™ powder contains the active component potassium peroxymonosulfate (KHSO5), also known as potassium monopersulfate. The oxidizing power of Oxone™ is derived from this peracid chemistry, making it one of the strongest oxidants available on the market. / Oxone™ is used worldwide in a variety of consumer and industrial applications. / Applications / Pool and spa / Water treatment / Pulp and paper / Home care / Electronics / Denture cleaners / Odor control / Disinfection.
- ^ Spiro, M. (1979). "The standard potential of the peroxosulphate/sulphate couple". Electrochimica Acta. 24 (3): 313–314. doi:10.1016/0013-4686(79)85051-3. ISSN 0013-4686.[non-primary source needed]
- ^ Travis, B. R.; Ciaramitaro, B. P. & Borhan, B. (September 30, 2002). "Preparation of Purified KHSO5·H2O and nBu4NHSO5 from Oxone by Simple and Efficient Methods". Eur. J. Org. Chem.: 3429–3434. doi:10.1002/1099-0690(200210)2002:20<3429::AID-EJOC3429>3.0.CO;2-D. Retrieved November 3, 2025.
{{cite journal}}: CS1 maint: multiple names: authors list (link) The article has been made available by an author, at this link. - ^ a b Jakob, Harald; Leininger, Stefan; Lehmann, Thomas; Jacobi, Sylvia & Gutewort, Sven (July 15, 2007). "Peroxo Compounds, Inorganic". In Ley, Claudia (ed.). Ullmann's Encyclopedia of Industrial Chemistry. Weinheim, Germany: Wiley-VCH. doi:10.1002/14356007.a19_177.pub2. Retrieved November 3, 2025.
{{cite encyclopedia}}: CS1 maint: multiple names: authors list (link) - ^ Peroxy Compounds Human Health and Ecological Draft Risk Assessment DP 455445, 455446 (Report). United States Environmental Protection Agency. March 11, 2020. p. 9-10. Retrieved September 24, 2021.
- ^ Wacławek, Stanisław; Lutze, Holger V.; Grübel, Klaudiusz; Padil, Vinod V.T.; Černík, Miroslav; Dionysiou, Dionysios. D. (December 15, 2017). "Peroxy Compounds Human Health and Ecological Draft Risk Assessment DP 455445, 455446". Chemical Engineering Journal. 330: 44–62. doi:10.1016/j.cej.2017.07.132.
- ^ TFP Staff (Anon.) (November 30, 2018). "ABC's of Pool Water Chemistry". Pool School. Unknown location: Trouble Free Pool (TFP). Retrieved November 3, 2025.
Potassium monopersulfate (a common non-chlorine shock) will show up on [ferrous ammonium sulfate, N,N′-diethyl-p-phenylenediamine,] FAS-DPD chlorine tests as [combined chlorine,] CC. There is a special reagent you can get to neutralize potassium monopersulfate so you can get a true CC reading.
See also the same content at the organisation's website, linked here. - ^ For a literature starting point for the FAS-DPD test, see Moberg, Ludvig & Karlberg, Bo (2000). "An Improved N,N′-diethyl-p-phenylenediamine (DPD) Method for the Determination of Free Chlorine Based on Multiple Wavelength Detection". Analytica Chimica Acta. 407 (1–2): 127–133. doi:10.1016/S0003-2670(99)00780-1. ISSN 0003-2670. Retrieved November 3, 2025.
{{cite journal}}: CS1 maint: multiple names: authors list (link)[non-primary source needed] - ^ Mundy, B.P.; Ellerd, M.G. & Favaloro Jr., F.G. (2005). "Oxone®". Name Reactions and Reagents in Organic Synthesis (2nd ed.). Hoboken, NJ: John Wiley & Sons. p. 828. ISBN 9780471739869. Retrieved November 3, 2025.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Travis, Benjamin R.; Sivakumar, Meenakshi; Hollist, G. Olatunji & Borhan, Babak (2003). "Facile Oxidation of Aldehydes to Acids and Esters with Oxone". Organic Letters. 5 (7): 1031–4. doi:10.1021/ol0340078. PMID 12659566.[non-primary source needed]
- ^ Adam, W.; Saha-Moller, C.; Zhao, C.-G. (2004). "Dioxirane Epoxidation of Alkenes". Org. React. 61: 219. doi:10.1002/0471264180.or061.02.
- ^ Adam, W.; Zhao, C.-G.; Jakka, K. (2007). "Dioxirane Oxidations of Compounds other than Alkenes". Org. Reactions. 69: 1. doi:10.1002/0471264180.or069.01.
- ^ Frigerio, M.; Santagostino, M.; Sputore, S. (1999). "A User-Friendly Entry to 2-Iodoxybenzoic Acid (IBX)". J. Org. Chem. 64 (12): 4537–4538. doi:10.1021/jo9824596.[non-primary source needed]
- ^ Bell, Thomas W.; Cho, Young-Moon; Firestone, Albert; Healy, Karin; Liu, Jia; Ludwig, Richard; Rothenberger, Scott D. (1990). "9-n-Butyl-1,2,3,4,5,6,7,8-Octahydroacridin-4-ol". Organic Syntheses. 69: 226. doi:10.15227/orgsyn.069.0226.
- ^ McCarthy, James R.; Matthews, Donald P.; P. Paolini, John (1995). "Reaction of Sulfoxides with Diethylaminosulfur Trifluoride". Organic Syntheses. 72: 209. doi:10.15227/orgsyn.072.0209.
- ^ Springer, E. L.; McSweeny, J. D. (1993). "Treatment of softwood kraft pulps with peroxymonosulfate before oxygen delignification". TAPPI Journal. 76 (8): 194–199. ISSN 0734-1415. Archived from the original on September 29, 2011. Retrieved May 14, 2011.

