Crown ether
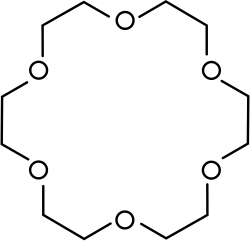
A crown ether is a type of organic compound. They are large rings of atoms where oxygen replaces carbon in a repeating pattern.[1] Related compounds that have nitrogen or sulfur as part of the pattern are called aza-crown ethers and thia-crown ethers.
Crown ethers were discovered by Charles J. Pedersen. Because of this discovery, Pedersen shared the 1987 Nobel Prize in Chemistry with two other researchers, Donald J. Cram who also worked on crown ethers, and Jean-Marie Lehn who worked with similar molecules called cryptands.[2]
Crown ethers are common ligands. They form coordination complexes with different metal cations depending on their size and shape. These complexes act like ionophores, allowing salts to dissolve in solvents that can't dissolve the non-complexed ion.
The most important group of crown ethers are the oligomers of ethylene oxide, particularly 12-crown-4, 15-crown-5, and 18-crown-6. Another important class replaces one or more ethylene oxides with catechols, making benzo crown ethers like dibenzo-18-crown-6.
Preparation
[change | change source]Crown ethers can be made by the Williamson ether synthesis. This reaction uses a diol or polyether and a bis(chloroalkyl) ether. They can also be made from oligomerisation of ethylene oxide, usually in solution with a cation that prefers a specific oligomer to guide the structure.
Naming
[change | change source]The IUPAC systematic names of crown ethers are usually very long. To shorten these names, chemists who study crown ethers use common names of the form (total number of atoms)-crown-(number of oxygen atoms). For example, the crown ether with 18 atoms, including 6 oxygen atoms, is called 18-crown-6.
Sources
[change | change source]- ↑ IUPAC, Compendium of Chemical Terminology, 5th ed. (the "Gold Book") (2025). Online version: (2006–) "crown". doi:10.1351/goldbook.C01421
- ↑ "The 1987 Nobel Prize in Chemistry" (Press release). The Nobel Foundation. 1987-10-14. Retrieved 2026-01-20.
