WD40 repeat
| WD domain, G-beta repeat | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
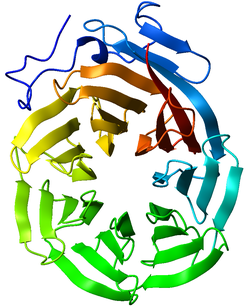 Ribbon diagram of the C-terminal WD40 domain of Tup1 (a transcriptional corepressor in yeast), which adopts a 7-bladed beta-propeller fold. Ribbon is colored from blue (N-terminus) to red (C-terminus).[1] | |||||||||||
| Identifiers | |||||||||||
| Symbol | WD40 | ||||||||||
| Pfam | PF00400 | ||||||||||
| Pfam clan | CL0186 | ||||||||||
| InterPro | IPR001680 | ||||||||||
| PROSITE | PDOC00574 | ||||||||||
| SCOP2 | 1gp2 / SCOPe / SUPFAM | ||||||||||
| CDD | cd00200 | ||||||||||
| |||||||||||
The WD40 repeat (also known as the WD or beta-transducin repeat) is a short structural motif of approximately 40 amino acids, often terminating in a tryptophan-aspartic acid (W-D) dipeptide.[2] Tandem copies of these repeats typically fold together to form a type of circular solenoid protein domain called the WD40 domain.
Structure
[edit]WD40 domain-containing proteins have 4 to 16 repeating units, all of which are thought to form a circularised beta-propeller structure (see figure to the right).[3][4] The WD40 domain is composed of about 40 to 60[4] amino acids with a glycine and histidine dipeptide near the N-terminus and a tryptophan and aspartic acid dipeptide most commonly at the C-terminus. Two variable regions are present. The repeats typically form a four-stranded anti-parallel beta sheet or blade. These blades come together to form a propeller with the most common being a seven-bladed beta propeller. The blades interlock so that the last beta strand of one repeat forms with the first three of the next repeat to form the 3D blade structure.[3][4]
Function
[edit]WD40-repeat proteins are a large family found in all eukaryotes and are implicated in a variety of functions ranging from signal transduction and transcription regulation to cell cycle control, autophagy and apoptosis.[5] The underlying common function of all WD40-repeat proteins is coordinating multi-protein complex assemblies, where the repeating units serve as a rigid scaffold for protein interactions. The specificity of the proteins is determined by the sequences outside the repeats themselves. Examples of such complexes are G proteins (beta subunit is a beta-propeller), TAFII transcription factor, and E3 ubiquitin ligase.[3][4]
Examples
[edit]According to the initial analysis of the human genome WD40 repeats are the eighth largest family of proteins. In all 277 proteins were identified to contain them.[6] Human genes encoding proteins containing this domain include:
- AAAS, AAMP, AHI1, AMBRA1, APAF1, ARPC1A, ARPC1B, ATG16L1,
- BOP1, BRWD1, BRWD3, BTRC, BUB3,
- C6orf11, CDC20, CDC40, CDRT1, CHAF1B, CIAO1, CIRH1A, COPA, COPB2, CORO1A, CORO1B, CORO1C, CORO2A, CORO2B, CORO6, CORO7, CSTF1,
- DDB2, DENND3, DMWD, DMXL1, DMXL2, DNAI1, DNAI2, DNCI1, DTL, DYNC1I1, DYNC1I2, EDC4,
- EED, EIF3S2, ELP2, EML1, EML2, EML3, EML4, EML4-ALK, EML5, ERCC8,
- FBXW10, FBXW11, FBXW2, FBXW4, FBXW5, FBXW7, FBXW8, FBXW9, FZR1,
- GBL, GEMIN5, GNB1, GNB1L, GNB2, GNB2L1, GNB3, GNB4, GNB5, GRWD1, GTF3C2,
- HERC1, HIRA, HZGJ,
- IFT121, IFT122, IFT140, IFT172, IFT80, IQWD1,
- KATNB1, KIAA1336, KIF21A, KIF21B, KM-PA-2,
- KEAP1,
- LLGL1, LLGL2, LRBA, LRRK1, LRRK2, LRWD1, LYST,
- MAPKBP1, MED16, MORG1,
- NBEA, NBEAL1, NEDD1, NLE1, NSMAF, NUP37, NUP43, NWD1,
- PAAF1, PAFAH1B1, PAK1IP1, PEX7, PHIP, PIK3R4, PLAA, PLRG1, PPP2R2A, PPP2R2B, PPP2R2C, PPP2R2D, PPWD1, PREB, PRPF19, PRPF4, PWP1, PWP2,
- RAE1, RPTOR, RBBP4, RBBP5, RBBP7, RFWD2, RFWD3, RRP9,
- SCAP, SEC13, SEC31A, SEC31B, SEH1L, SHKBP1, SMU1, SPAG16, SPG, STRAP, STRN, STRN3, STRN4, STXBP5, STXBP5L,
- TAF5, TAF5L, TBL1X, TBL1XR1, TBL1Y, TBL2, TBL3, TEP1, THOC3, THOC6, TLE1, TLE2, TLE3, TLE4, TLE6, TRAF7, TSSC1, TULP4, TUWD12,
- UTP15, UTP18,
- WAIT1, WDF3, WDFY1, WDFY2, WDFY3, WDFY4, WDHD1, WDR1, WDR10, WDR11, WDR12, WDR13, WDR16, WDR17, WDR18, WDR19, WDR20, WDR21A, WDR21C, WDR22, WDR23, WDR24, WDR25, WDR26, WDR27, WDR3, WDR31, WDR32, WDR33, WDR34, WDR35, WDR36, WDR37, WDR38, WDR4, WDR40A, WDR40B, WDR40C, WDR41, WDR42A, WDR42B, WDR43, WDR44, WDR46, WDR47, WDR48, WDR49, WDR5, WDR51A, WDR51B, WDR52, WDR53, WDR54, WDR55, WDR57, WDR59, WDR5B, WDR6, WDR60, WDR61, WDR62, WDR63, WDR64, WDR65, WDR66, WDR67, WDR68, WDR69, WDR7, WDR70, WDR72, WDR73, WDR74, WDR75, WDR76, WDR77, WDR78, WDR79, WDR8, WDR81, WDR82, WDR85, WDR86, WDR88, WDR89, WDR90, WDR91, WDR92, WDSOF1, WDSUB1, WDTC1, WSB1, WSB2,
- ZFP106
| WDR gene | other gene names | NCBI Entrez Gene ID |
Human disease associated with mutations |
|---|---|---|---|
| WDR1 | AIP1; NORI-1; HEL-S-52 | 9948 | |
| WDR2 | CORO2A; IR10; CLIPINB | 7464 | |
| WDR3 | DIP2; UTP12 | 10885 | |
| WDR4 | TRM82; TRMT82 | 10785 | |
| WDR5 | SWD3; BIG-3; CFAP89 | 11091 | |
| WDR6 | 11180 | ||
| WDR7 | TRAG; KIAA0541; Rabconnectin 3 beta | 23335 | |
| WDR8 | WRAP73 | 49856 | |
| WDR9 | BRWD1; N143; C21orf107 | 54014 | |
| WDR10 | IFT122; CED; SPG; CED1; WDR10p; WDR140 | 55764 | Sensenbrenner syndrome |
| WDR11 | DR11; HH14; BRWD2; WDR15 | 55717 | Kallmann syndrome |
| WDR12 | YTM1 | 55759 | |
| WDR13 | MG21 | 64743 | |
| WDR14 | GNB1L; GY2; FKSG1; WDVCF; DGCRK3 | 54584 | |
| WDR15 | WDR11 | ||
| WDR16 | CFAP52; WDRPUH | 146845 | |
| WDR17 | 116966 | ||
| WDR18 | Ipi3 | 57418 | |
| WDR19 | ATD5; CED4; DYF-2; ORF26; Oseg6; PWDMP; SRTD5; IFT144; NPHP13 | 57728 | Sensenbrenner syndrome, Jeune syndrome |
| WDR20 | DMR | 91833 | |
| WDR21 | DCAF4; WDR21A | 26094 | |
| WDR22 | DCAF5; BCRG2; BCRP2 | 8816 | |
| WDR23 | DCAF11; GL014; PRO2389 | 80344 | |
| WDR24 | JFP7; C16orf21 | 84219 | |
| WDR25 | C14orf67 | 79446 | |
| WDR26 | CDW2; GID7; MIP2 | 80232 | |
| WDR27 | 253769 | ||
| WDR28 | GRWD1; CDW4; GRWD; RRB1 | 83743 | |
| WDR29 | SPAG16; PF20 | 79582 | |
| WDR30 | ATG16L1; IBD10; APG16L; ATG16A; ATG16L | 55054 | Crohn’s disease |
| WDR31 | 114987 | ||
| WDR32 | DCAF10 | 79269 | |
| WDR33 | NET14; WDC146 | 55339 | |
| WDR34 | DIC5; FAP133; SRTD11 | 89891 | Jeune syndrome |
| WDR35 | CED2; IFTA1; SRTD7; IFT121 | 57539 | Sensenbrenner syndrome |
| WDR36 | GLC1G; UTP21; TAWDRP; TA-WDRP | 134430 | Primary Open Angle Glaucoma |
| WDR37 | 22884 | ||
| WDR38 | 401551 | ||
| WDR39 | CIAO1; CIA1 | 9391 | |
| WDR40A | DCAF12; CT102; TCC52; KIAA1892 | 25853 | |
| WDR41 | MSTP048 | 55255 | |
| WDR43 | UTP5; NET12 | 23160 | |
| WDR44 | RPH11; RAB11BP | 54521 | |
| WDR45 | JM5; NBIA4; NBIA5; WDRX1; WIPI4; WIPI-4 | 11152 | Beta-propeller protein-associated neurodegeneration (BPAN) |
| WDR46 | UTP7; BING4; FP221; C6orf11 | 9277 | |
| WDR47 | NEMITIN; KIAA0893 | 22911 | |
| WDR48 | P80; UAF1; SPG60 | 57599 | |
| WDR49 | 151790 | ||
| WDR50 | UTP18; CGI-48 | 51096 | |
| WDR52 | CFAP44 | 55779 | |
| WDR53 | 348793 | ||
| WDR54 | 84058 | ||
| WDR55 | 54853 | ||
| WDR56 | IFT80; ATD2; SRTD2 | 57560 | Jeune syndrome |
| WDR57 | SNRNP40; SPF38; PRP8BP; HPRP8BP; PRPF8BP | 9410 | |
| WDR58 | THOC6; BBIS; fSAP35 | 79228 | |
| WDR59 | FP977 | 79726 | |
| WDR60 | SRPS6; SRTD8; FAP163 | 55112 | Jeune syndrome |
| WDR61 | SKI8; REC14 | 80349 | |
| WDR62 | MCPH2; C19orf14 | 284403 | microcephaly |
| WDR63 | DIC3; NYD-SP29 | 126820 | |
| WDR64 | 128025 | ||
| WDR65 | CFAP57; VWS2 | 149465 | Van der Woude syndrome |
| WDR66 | CaM-IP4 | 144406 | |
| WDR67 | TBC1D31; Gm85 | 93594 | |
| WDR68 | DCAF7; AN11; HAN11; SWAN-1 | 10238 | |
| WDR69 | DAW1; ODA16 | 164781 | |
| WDR70 | 55100 | ||
| WDR71 | PAAF1; PAAF; Rpn14 | 80227 | |
| WDR72 | AI2A3 | 256764 | Amelogenesis imperfecta |
| WDR73 | HSPC264 | 84942 | |
| WDR74 | 54663 | ||
| WDR75 | NET16; UTP17 | 84128 | |
| WDR76 | CDW14 | 79968 | |
| WDR77 | p44; MEP50; MEP-50; HKMT1069; Nbla10071; p44/Mep50 | 79084 | |
| WDR78 | DIC4 | 79819 | |
| WDR79 | WRAP53; DKCB3; TCAB1 | 55135 | |
| WDR80 | ATG16L; ATG16B | 89849 | |
| WDR81 | CAMRQ2; PPP1R166 | 124997 | cerebellar ataxia, mental retardation, and dysequilibrium syndrome-2 |
| WDR82 | SWD2; MST107; WDR82A; MSTP107; PRO2730; TMEM113; PRO34047 | 80335 | |
| WDR83 | MORG1 | 84292 | |
| WDR84 | PAK1IP1; PIP1; MAK11 | 55003 | |
| WDR85 | DPH7; RRT2; C9orf112 | 92715 | |
| WDR86 | 349136 | ||
| WDR87 | NYD-SP11 | 83889 | |
| WDR88 | PQWD | 126248 | |
| WDR89 | MSTP050; C14orf150 | 112840 | |
| WDR90 | C16orf15; C16orf16; C16orf17; C16orf18; C16orf19 | 197335 | |
| WDR91 | HSPC049 | 29062 | |
| WDR92 | MONAD | 116143 | |
| WDR93 | 56964 | ||
| WDR94 | AMBRA1; DCAF3 | 55626 | |
| WDR96 | CFAP43; C10orf79 | 80217 |
See also
[edit]- Beta-propeller
- Tomosyn, a protein two WD40 domains
- Protein tandem repeats
References
[edit]- ^ PDB: 1erj; Sprague ER, Redd MJ, Johnson AD, Wolberger C (June 2000). "Structure of the C-terminal domain of Tup1, a corepressor of transcription in yeast". EMBO J. 19 (12): 3016–27. doi:10.1093/emboj/19.12.3016. PMC 203344. PMID 10856245.
- ^ Neer EJ, Schmidt CJ, Nambudripad R, Smith TF (September 1994). "The ancient regulatory-protein family of WD-repeat proteins". Nature. 371 (6495): 297–300. Bibcode:1994Natur.371..297N. doi:10.1038/371297a0. PMID 8090199. S2CID 600856.
- ^ a b c Smith TF, Gaitatzes C, Saxena K, Neer EJ (May 1999). "The WD40 repeat: a common architecture for diverse functions". Trends Biochem. Sci. 24 (5): 181–5. doi:10.1016/S0968-0004(99)01384-5. PMID 10322433.
- ^ a b c d Li D, Roberts R (December 2001). "WD-repeat proteins: structure characteristics, biological function, and their involvement in human diseases". Cell. Mol. Life Sci. 58 (14): 2085–97. doi:10.1007/PL00000838. PMC 11337334. PMID 11814058. S2CID 20646422.
- ^ Stirnimann CU, Petsalaki E, Russell RB, Müller CW (May 2010). "WD40 proteins propel cellular networks". Trends Biochem. Sci. 35 (10): 565–74. doi:10.1016/j.tibs.2010.04.003. PMID 20451393.
- ^ Lander ES, Linton LM, Birren B, et al. (February 2001). "Initial sequencing and analysis of the human genome" (PDF). Nature. 409 (6822): 860–921. doi:10.1038/35057062. PMID 11237011.
External links
[edit]- Eukaryotic Linear Motif resource motif class LIG_APCC_Dbox_1
- Eukaryotic Linear Motif resource motif class LIG_APCC_KENbox_2
- Eukaryotic Linear Motif resource motif class LIG_COP1
- Eukaryotic Linear Motif resource motif class LIG_CRL4_Cdt2_1
- Eukaryotic Linear Motif resource motif class LIG_CRL4_Cdt2_2
- Eukaryotic Linear Motif resource motif class LIG_EH1_1
- Eukaryotic Linear Motif resource motif class LIG_GLEBS_BUB3_1
- Eukaryotic Linear Motif resource motif class LIG_RAPTOR_TOS_1
- Eukaryotic Linear Motif resource motif class LIG_SCF_FBW7_1
- Eukaryotic Linear Motif resource motif class LIG_SCF_FBW7_2
- Eukaryotic Linear Motif resource motif class LIG_SCF-TrCP1_1
- Eukaryotic Linear Motif resource motif class LIG_WRPW_1
- Eukaryotic Linear Motif resource motif class LIG_WRPW_2
- Eukaryotic Linear Motif resource motif class TRG_ER_diArg_1
- Eukaryotic Linear Motif resource motif class TRG_ER_diLys_1
- Eukaryotic Linear Motif resource motif class TRG_Golgi_diPhe_1
- Eukaryotic Linear Motif resource motif class TRG_PTS2
