Coeliac disease
| Coeliac disease | |
|---|---|
| Other names | Coeliac sprue, nontropical sprue, endemic sprue, gluten enteropathy |
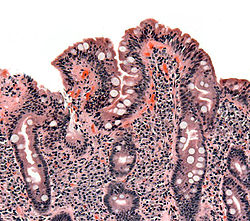 | |
| Biopsy of small bowel showing coeliac disease manifested by blunting of villi, crypt hyperplasia, and lymphocyte infiltration of crypts | |
| Pronunciation | |
| Specialty | Gastroenterology, internal medicine |
| Symptoms | None or non-specific, diarrhoea, malabsorption, and weight loss. |
| Complications | Iron-deficiency anemia, osteoporosis, infertility, cancers, and nutritional deficiencies. |
| Usual onset | Any age |
| Duration | Lifelong |
| Causes | Reaction to gluten |
| Risk factors | Genetic predisposition and environmental factors |
| Diagnostic method | Blood tests, intestinal biopsies, and clinical criteria |
| Differential diagnosis | Inflammatory bowel disease, intestinal parasites, irritable bowel syndrome, cystic fibrosis |
| Treatment | Gluten-free diet |
| Frequency | Between 1 in 50 and 1 in 200 |
Coeliac disease (Commonwealth English) or celiac disease (American English) is a chronic autoimmune disease, mainly affecting the small intestine, and is caused by the consumption of gluten. Coeliac disease causes a wide range of symptoms and complications that can affect multiple organs outside of the gastrointestinal tract.
The symptoms of coeliac disease can be divided into two subtypes, classic and non-classic. The classic form of the disease can affect any age group, but is usually diagnosed in early childhood and causes symptoms of malabsorption such as weight loss, diarrhoea, and stunted growth. Non-classic coeliac disease is more commonly seen in adults and is characterized by vague abdominal symptoms and complications in organs outside of the gastrointestinal tract, such as bone disease, anemia, and other consequences of nutritional deficiencies.
Coeliac disease is caused by an abnormal immune system response to gluten, found in wheat and other grains such as barley and rye. When an individual with a genetic predisposition to coeliac disease consumes gluten, it triggers an inflammatory response in the small intestine, damaging the intestinal lining, leading to malabsorption. The development of coeliac disease is believed to be influenced by other environmental factors, such as infections.
Diagnosis is typically made by a combination of blood antibody tests and intestinal biopsies, helped by specific genetic testing.[1] Diagnosis is not always straightforward.[2] About 10% of the time, the autoantibodies in the blood are negative,[3][4] and many people have only minor intestinal changes with normal villi.[5] People may have severe symptoms and they may be investigated for years before a diagnosis is achieved.[6][7] As a result of screening, the diagnosis is increasingly being made in people who have no symptoms.[8] Evidence regarding the effects of screening, however, is as of 2017, insufficient to determine its usefulness.[9] While the disease is caused by a permanent intolerance to gluten proteins,[1] it is distinct from wheat allergy, which is much rarer.[10][11]
The only known effective treatment is a strict lifelong gluten-free diet, which leads to recovery of the intestinal lining (mucous membrane), improves symptoms, and reduces the risk of developing complications in most people.[12] If untreated, it may result in cancers such as intestinal lymphoma, and a slightly increased risk of early death.[13] Rates vary between different regions of the world, from as few as 1 in 300 to as many as 1 in 40, with an average of between 1 in 100 and 1 in 170 people.[14] It is estimated that 80% of cases remain undiagnosed, usually because of minimal or absent gastrointestinal complaints and lack of knowledge of symptoms and diagnostic criteria.[15][6][16] Coeliac disease is slightly more common in women than in men.[17]
Signs and symptoms
[edit]Coeliac disease causes a wide range of symptoms and complications that can involve several different organs.[18] The presentation of coeliac disease can be classified as classic, non-classic, and subclinical.[19] Classic coeliac disease is commonly seen in young children, but can affect any age group, and is characterized by malabsorption manifesting as diarrhoea, weight loss, and failure to thrive.[18][20] Non-classic coeliac disease is seen more often in adults and symptoms primarily manifest outside of the intestine (extraintestinal).[20] Many undiagnosed individuals who consider themselves asymptomatic are, in fact, not, but rather have become accustomed to living in a state of chronically compromised health. After starting a gluten-free diet and subsequent improvement becomes evident, such individuals are often able to retrospectively recall and recognise prior symptoms of their untreated disease that they had mistakenly ignored.[21][22][16]
Gastrointestinal
[edit]Diarrhoea that is characteristic of coeliac disease is chronic, sometimes pale, of large volume, and abnormally foul in odor. Other symptoms of coeliac disease include abdominal pain, cramping, bloating with abdominal distension, and mouth ulcers.[23][24] As the bowels become more damaged, lactose intolerance can develop.[25] Frequently, the symptoms are ascribed to irritable bowel syndrome (IBS), only later to be recognised as coeliac disease.
Extraintestinal manifestations
[edit]Coeliac disease is a systemic disorder, meaning it affects the entire body. Although many common symptoms of the disease are related to the gastrointestinal tract, those with coeliac disease may also experience symptoms and complications in other organs, known as extraintestinal manifestations.[26] These manifestations may be related to malabsorption or systemic inflammation.[27] Common extraintestinal manifestations of coeliac disease include headaches, fatigue, brain fog, muscle pain, and joint pain.[27][28]
The changes in the bowel reduce its ability to absorb nutrients, minerals, and vitamins:[29]
- Malabsorption may cause weight loss (or failure to thrive or stunted growth in children) and fatigue or lack of energy.[24]
- Anaemia can develop due to deficiencies in folic acid and vitamin B12, or iron deficiency anaemia.[27]
- Calcium and vitamin D malabsorption (and compensatory secondary hyperparathyroidism) may cause osteopenia (decreased mineral content of the bone) or osteoporosis (bone weakening and risk of fragility fractures).[27][30]
- Higher rates of infertility have been shown in those with coeliac disease, possibly due to deficiencies in zinc and selenium.[27]
- A small proportion of people have abnormal coagulation because of vitamin K deficiency and are at a slight risk of abnormal bleeding.[27]
Miscellaneous
[edit]Coeliac disease has been linked with many conditions. In many cases, it is unclear whether the gluten-induced bowel disease is a causative factor or whether these conditions share a common predisposition.[27][28]
- IgA deficiency is present in 2-2.5% of people with coeliac disease, and is itself associated with an increased risk of coeliac disease.[31]
- Dermatitis herpetiformis, an itchy cutaneous condition that has been linked to a transglutaminase enzyme in the skin. Enteropathy is commonly seen in dermatitis herpetiformis; however, gastrointestinal symptoms are rare.[28]
- Growth failure and/or pubertal delay in later childhood can occur even without obvious bowel symptoms or severe malnutrition.[23]
- Reproductive disorders such as delayed puberty, lack of menstruation, early menopause, infertility, miscarriage and intrauterine growth restriction are increased in coeliac disease.[28]
- Hyposplenism (a small and underactive spleen) can occur and may predispose to infection given the role of the spleen in the immune system.[28]
- Abnormal liver function tests (randomly detected on blood tests) may be seen.[24]
- Depression, anxiety and other mental health disorders.[27]
Coeliac disease is associated with several other medical conditions, many of which are autoimmune disorders: diabetes mellitus type 1, hypothyroidism, primary biliary cholangitis, microscopic colitis, gluten ataxia, psoriasis, vitiligo, autoimmune hepatitis, primary sclerosing cholangitis, and more.[24][28]
Complications
[edit]Coeliac disease leads to an increased risk of both adenocarcinoma and lymphoma of the small bowel (enteropathy-associated T-cell lymphoma or other non-Hodgkin lymphomas) within the first year of diagnosis.[24] Whether a gluten-free diet brings this risk back to baseline is unclear.[32] Long-standing and untreated disease can rarely lead to other complications, such as ulcerative jejunitis (ulcer formation of the small bowel).[33]
Causes
[edit]Coeliac disease is caused by an inflammatory reaction to gliadins and glutenins (gluten proteins)[34] found in wheat and to similar proteins found in the crops of the tribe Triticeae (which includes other common grains such as barley and rye) and to the tribe Aveneae (oats).[35] Wheat subspecies (such as spelt, durum, and Kamut) and wheat hybrids (such as triticale) also cause symptoms of coeliac disease.[36]
A small number of people with coeliac disease react to oats. Sensitivity to oats in coeliac disease may be due to cross-contamination of oats and other foods with gluten, differences between gluten content, immunoreactivity, and genetic variability seen between oat cultivars or dietary intolerance to oats.[37][38] Most people with coeliac disease do not have adverse reactions to uncontaminated or 'pure' oats, however clinical guidelines differ on whether those with coeliac disease should consume oats.[39][40]
Other cereals such as maize, millet, sorghum, teff, rice, and wild rice are safe for people with coeliac disease to consume, as well as non-cereals such as amaranth, quinoa, and buckwheat. Noncereal carbohydrate-rich foods such as potatoes and bananas do not contain gluten and do not trigger symptoms.[41][42]
Risk modifiers
[edit]Environmental factors such as infections, geographic latitude, birth weight, antibiotic use, intestinal microbiota, socioeconomic status, hygiene, breastfeeding, and the timing of introduction of gluten into an infant's diet are theorized to contribute to the development of coeliac disease in genetically predisposed individuals.[43][34][20] The consumption of gluten and timing of introduction in a baby's life does not appear to increase the risk of coeliac disease, however in those who are genetically predisposed to coeliac disease, large amounts of gluten early in life, may increase the risk of developing coeliac disease.[44][45]
Mechanism
[edit]Coeliac disease appears to be multifactorial, both in that more than one genetic factor can cause the disease and in that more than one factor is necessary for the disease to manifest in a person.[46]
Almost all people (90%) with coeliac disease have either the variant HLA-DQ2 allele or (less commonly) the HLA-DQ8 allele.[19] However, about 40% of people without coeliac disease have also inherited either of these alleles.[47] This suggests that additional factors are needed for coeliac disease to develop; that is, the predisposing HLA risk allele is necessary but not sufficient to develop coeliac disease. Furthermore, around 5% of those people who do develop coeliac disease do not have typical HLA-DQ2 or HLA-DQ8 alleles.[19][48]
Genetics
[edit]The vast majority of people with coeliac have one of two types (out of seven) of the HLA-DQ protein.[19] HLA-DQ is part of the MHC class II antigen-presenting receptor[50] (also called the human leukocyte antigen) system and is used by the immune system to distinguish between the body’s own cells and others.[51][52] The two subunits of the HLA-DQ protein are encoded by the HLA-DQA1 and HLA-DQB1 genes, located on the short arm of chromosome 6.[53]
There are seven HLA-DQ variants (DQ2 and DQ4–DQ9). Over 95% of people with coeliac disease have the isoform of DQ2 or DQ8, which is inherited in families. The reason these genes produce an increase in the risk of coeliac disease is that the receptors formed by these genes bind to gliadin peptides more tightly than other forms of the antigen-presenting receptor. Therefore, these forms of the receptor are more likely to activate T lymphocytes and initiate the autoimmune process.[53]

Most people with coeliac bear a two-gene HLA-DQ2 haplotype called DQ2.5. This haplotype is composed of two adjacent gene alleles, DQA1*0501 and DQB1*0201, which encode the two subunits, DQ α5 and DQ β2.[54][55] In most individuals, this DQ2.5 isoform is encoded by one of two chromosomes 6 inherited from parents (DQ2.5cis). Most coeliacs inherit only one copy of this DQ2.5 haplotype, while some inherit it from both parents; the latter are especially at risk of coeliac disease as well as being more susceptible to severe complications.[56] The frequency of coeliac disease haplotypes can vary by geography.[46][57]
Some individuals inherit DQ2.5 from one parent and an additional portion of the haplotype (either DQB1*02 or DQA1*05) from the other parent, increasing risk. Less commonly, some individuals inherit the DQA1*05 allele from one parent and the DQB1*02 from the other parent (DQ2.5trans), and these individuals are at similar risk of coeliac disease as those with a single DQ2.5-bearing chromosome 6.[56][53] Among those with coeliac disease who do not have DQ2.5 (cis or trans) or DQ8 (encoded by the haplotype DQA1*03:DQB1*0302), 2-5% have the DQ2.2 isoform, and the remaining 2% lack DQ2 or DQ8.[56]
Other genetic factors have been repeatedly reported in coeliac disease; however, involvement in the disease has variable geographic recognition. Only the HLA-DQ loci show a consistent involvement over the global population. Many of the loci detected have been found in association with other autoimmune diseases.[48]
The prevalence of the HLA-DQ2 genotype and gluten consumption has increased over time. Since untreated coeliac disease can cause serious health problems and affect fertility, it would be expected that HLA-DQ2 and HLA-DQ8 would become less common. The opposite is true—they are most common in areas where gluten-rich foods have been eaten for thousands of years.[58] The HLA-DQ2 gene may have been genetically favoured in the past because it helps protect against tooth decay.[57][59]
Prolamins
[edit]Most of the proteins in food responsible for the immune reaction in coeliac disease are prolamins. These are storage proteins rich in proline (prol-) and glutamine (-amin) that dissolve in alcohols and are resistant to proteases and peptidases of the gut.[60] Prolamins are found in cereal grains with different grains having different but related prolamins: wheat (gliadin), barley (hordein), rye (secalin) and oats (avenin).[35][19]

Membrane leaking permits[clarification needed] peptides of gliadin that stimulate two levels of the immune response: the innate response and the adaptive (T-helper cell-mediated) response. One protease-resistant peptide from α-gliadin contains a region that stimulates lymphocytes and results in the release of interleukin-15. This innate response to gliadin results in immune-system signalling that attracts inflammatory cells and increases the release of inflammatory chemicals.[8][needs update?]
The response to the 33mer occurs in most coeliacs who have a DQ2 isoform. This peptide, when altered by intestinal transglutaminase, has a high density of overlapping T-cell epitopes. This increases the likelihood that the DQ2 isoform will bind, and stay bound to, peptide when recognised by T-cells.[61]
Tissue transglutaminase
[edit]
Tissue transglutaminase modifies gluten peptides into a form that may stimulate the immune system more effectively.[60] These peptides are modified by tTG in two ways, deamidation or transamidation.[62]
Deamidation is the reaction by which a glutamate residue is formed by cleavage of the epsilon-amino group of a glutamine side chain.[63] Transamidation is the cross-linking of a glutamine residue from the gliadin peptide to a lysine residue of tTg in a reaction that is catalysed by the transglutaminase.[62] Crosslinking may occur either within or outside the active site of the enzyme. The latter case yields a permanently covalently linked complex between the gliadin and the tTg. This results in the formation of new epitopes believed to trigger the primary immune response by which the autoantibodies against tTg develop.[64]
Stored biopsies from people with suspected coeliac disease have revealed that autoantibody deposits in the subclinical coeliacs are detected prior to clinical disease.[60]
Villous atrophy and malabsorption
[edit]The inflammatory process, mediated by T cells, leads to disruption of the structure and function of the small bowel's mucosal lining and causes malabsorption as it impairs the body's ability to absorb nutrients from food.[48][47]
Alternative causes of this tissue damage have been proposed and involve the release of interleukin 15 and activation of the innate immune system by a shorter gluten peptide (p31–43/49).[60]
Diagnosis
[edit]The diagnosis of coeliac disease is often complicated by the variety in symptoms, overlap with other disorders, and lack of awareness in medical professionals, leading to a delay in the diagnosis being made.[65][21] A diagnosis may take more than a decade after symptoms develop, and most people with coeliac disease remain undiagnosed.[22][66] Delays in diagnosis can reduce quality of life, use more medical resources and increase risk of complications associated with the disease.[65][67][68]
Coeliac disease is diagnosed based on symptoms, blood tests, and biopsies of the small intestine.[65] To make an accurate diagnosis, an individual must be consuming gluten, as the reliability of biopsies and blood tests reduces if a person is on a gluten-free diet. In those who have already reduced their gluten intake, reintroducing gluten (gluten challenge) may be required to reach an accurate diagnosis.[69] Within months of eliminating gluten from one's diet, antibodies associated with coeliac disease decrease, meaning that gluten has to be reintroduced several weeks before diagnostic testing.[69][70]
Blood tests
[edit]
Current medical guidelines recommend testing tissue transglutaminase 2 immunoglobulin A (TTG IgA) in those with suspected coeliac disease.[71][72] Because IgA deficiency is more common in those with coeliac disease,[73] guidelines recommend testing for IgA deficiency as a part of the diagnostic workup for coeliac disease. If an individual with IgA deficiency is getting tested for coeliac disease, immunoglobulin G (IgG) based tests such as deamidated gliadin peptide IgG (DGP IgG) or endomysial antibody (EMA) can be used instead of IgA-based tests.[71][72] Antigliadin antibodies (AGA) and antireticulin antibodies (ARA) were historically used to test for coeliac disease, however due to the development of more accurate tests, they are no longer recommended.[43][73] Due to the risk of false positive or negative serological tests and the consequences of leaving coeliac disease untreated or introducing unnecessary dietary restrictions in the case of a false positive, biopsies are used to confirm the diagnosis regardless of blood tests.[43][72]
TG2 IgA has a high sensitivity (92.8%) and specificity (97.9%), is cost-efficient and widely available, making it the first choice for serological tests in the diagnosis of coeliac disease.[74][69] Despite this, performance of the TG2 IgA test differs between labs and no formal standardisation between assays exists.[21] The severity of small intestine damage generally correlates with the levels of TG2 IgA found in the blood, meaning that the sensitivity is lower in people who have less damage to their intestines.[74][73]
EMA has a lower sensitivity, but its specificity is near 100%.[74] Because of the high specificity, EMA can be used to confirm coeliac disease in those who have borderline TG2 IgA levels.[69] EMA testing is costly, hard to interpret and vulnerable to interobserver and inter-site variability.[21][75]
DGP IgG is used to evaluate coeliac disease in those with IgA-deficiency. Coeliac disease is more common in those with IgA-deficiency, so medical guidelines recommend that people being tested for coeliac disease are also tested for IgA-deficiency. Because IgA-based tests are unreliable in those with IgA deficiency, IgG-based tests are used instead. These include EMA IgG, DGP IgG, and TTG IgA, which are less accurate than IgA testing.[73][76]
A 2020 guideline by the European Society of Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) suggests biopsy can be avoided in children who have symptoms of coeliac disease, TTG IgA levels ten times higher than normal, and a positive EMA antibody. However there is not enough evidence to suggest that a nonbiopsy approach can be used in adults.[72]
Genetic testing is not needed to diagnose coeliac disease, but is sometimes used to clarify discrepancies between blood tests and histology. In those who have already started a gluten-free diet, HLA testing can help to determine whether a gluten challenge should be performed.[72]
Endoscopy
[edit]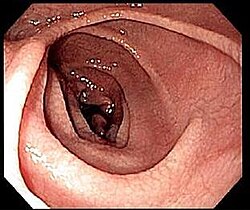
An upper endoscopy with biopsy of the duodenum (beyond the duodenal bulb) or jejunum is performed to obtain multiple samples from the duodenum.[72] Not all areas may be equally affected; if biopsies are taken from healthy bowel tissue, the result would be a false negative. Even in the same bioptic fragment, different degrees of damage may be present.[69]
Most people with coeliac disease have a small intestine that appears to be normal on endoscopy before the biopsies are examined.[77] Endoscopic features of coeliac disease include scalloping of the small bowel folds (pictured), fissures, a mosaic pattern to the mucosa, prominence of the submucosa blood vessels, and a nodular pattern to the mucosa.[35]
Capsule endoscopy (CE) allows identification of typical mucosal changes observed in coeliac disease and may be used as an alternative to endoscopy in those who cannot or do not want one.[43]
Pathology
[edit]The Marsh-Oberhuber classification is commonly used to assess the pathological changes seen in coeliac disease.[20] Marsh originally described three different stages of coeliac disease lesions in 1992. These three stages were updated in 1999 by Oberhuber to further classify stage three.[78][79] The Marsh classification is based on three histological features: intraepithelial lymphocytes count above 25/100 enterocytes (intraepithelial lymphocytosis), elongated crypts of Lieberkuhn (crypt hyperplasia), and shortening or absence of villi (villous atrophy). As these features can be seen in other disorders, they are not diagnostic for coeliac disease without serological or clinical indications.[78] Current guidelines do not recommend a repeat biopsy unless there is no improvement in the symptoms on a gluten free diet.[70]
| Type | Increased intraepithelial lymphocytes | Crypt hyperplasia | Villi |
|---|---|---|---|
| 0 (normal) | <40 lymphocytes/100 enterocytes | Normal | Normal |
| 1 (infiltrative) | >40 lymphocytes/100 enterocytes | ||
| 2 (hyperplastic) | Increased | ||
| 3a (destructive) | Mild atrophy | ||
| 3b (destructive) | Moderate atrophy | ||
| 3c (destructive) | Complete atrophy |
Gluten challenge
[edit]Currently, gluten challenge is no longer required to confirm the diagnosis in patients with intestinal lesions compatible with coeliac disease and a positive response to a gluten-free diet. A gluten challenge involves consuming over 10 grams of gluten a day for three months or until an individual tests positive for TG2 IgA.[74] Nevertheless, in some cases, a gluten challenge with a subsequent biopsy may be useful to support the diagnosis, for example, in people with positive HLA genetic testing who have negative blood antibodies and are already on a gluten-free diet.[43] Gluten challenge is discouraged before the age of 6 years and during pubertal growth.[74]
Differential diagnosis
[edit]The histopathological features associated with coeliac disease can arise from other conditions as well.[75] Differential diagnosis of negative coeliac blood tests and villous atrophy or increased inter-epithelial lymphocytes includes tropical sprue, eosinophilic gastroenteritis, lactose intolerance, lymphoma, Crohn’s disease, Helicobacter pylori, drug-induced enteropathy (azathioprine, methotrexate, mycophenolate, olmesartan, colchicinenon, non-steroidal anti-inflammatory drugs, and proton pump inhibitors), whipple disease, Giardiasis, radiation enteritis, tuberculosis, Zollinger–Ellison syndrome, collagenous sprue, common variable immunodeficiency, autoimmune enteropathy, HIV enteropathy, small intestinal bacterial overgrowth, and gastrinoma with acid hypersecretion.[77][35][79][75] If the histological changes improve with a gluten free diet despite negative coeliac disease blood tests a diagnosis of seronegative coeliac disease may be made.[35]
Positive blood tests for coeliac disease with a lack of changes in the bowels can be caused by errors in collecting blood for the test, recent infections, congestive heart failure, chronic liver disease, and hypergammaglobulinemia. Potential coeliac disease, formerly known as "latent coeliac disease" is diagnosed when there is positive coeliac blood tests, positive HLA genetic testing, and a lack of villous atrophy.[75][43]
Non-celiac gluten sensitivity (NCGS) is a functional disorder that causes intestinal and extraintestinal symptoms in response to gluten. The symptoms of NCGS are often similiar to those seen in coeliac disease, however they tend to have a more rapid onset and offset when compared to coeliac disease. The diagnosis of NCGS is made based on the exclusion of coeliac disease and wheat allergy, and a resolution of symptoms after adhering to a gluten free diet.[79][35]
Up to 30% of people redevelop or still have frequent symptoms after starting a gluten-free diet.[12] A careful interpretation of the symptomatic response is needed, as a lack of response in a person with coeliac disease may be due to continued ingestion of small amounts of gluten, either voluntary or inadvertent,[80] or be due to other commonly associated conditions such as small intestinal bacterial overgrowth (SIBO), lactose intolerance, fructose,[81] sucrose,[82] and sorbitol[83] malabsorption, exocrine pancreatic insufficiency,[84][85] and microscopic colitis,[85] among others. In untreated coeliac disease, these are often transient conditions derived from the intestinal damage.[82][83][86][87][88] They normally revert or improve several months after starting a gluten-free diet, but may need temporary interventions such as supplementation with pancreatic enzymes,[87][88] dietary restrictions of lactose, fructose, sucrose or sorbitol containing foods,[82][86] or treatment with oral antibiotics in the case of associated bacterial overgrowth.[88] In addition to gluten withdrawal, some people need to follow a low-FODMAPs diet or avoid consumption of commercial gluten-free products, which are usually rich in preservatives and additives (such as sulfites, glutamates, nitrates and benzoates) and might have a role in triggering functional gastrointestinal symptoms.[89]
Screening
[edit]There is debate as to the benefits of widespread screening measures for coeliac disease.[68] In 2017, the United States Preventive Services Task Force published a report found insufficient evidence to make a recommendation regarding screening for coeliac disease in those without symptoms.[9] Due to the lack of evidence that screening for coeliac disease in those without symptoms, clinical guidelines advise testing people based on symptoms and selective screening for certain populations at a higher risk of developing coeliac disease.[69][43]
| Testing recommended | Testing considered |
|---|---|
| Persistent unexplained gastrointestinal symptoms | Metabolic bone disease (reduced bone mineral density or osteomalacia) |
| Faltering growth | Unexplained neurological symptoms (such as peripheral neuropathy and ataxia) |
| Chronic fatigue | Fertility problems or recurrent miscarriage |
| Severe or persistent mouth ulcers | Persistently raised liver enzymes with unknown cause |
| Unexplained iron, vitaminB12, or folate deficiency | Dental enamel defects |
| At diagnosis of type 1 diabetes | Down syndrome |
| At diagnosis of an autoimmune thyroid disease | Turner syndrome |
| Irritable bowel syndrome in adults | |
| First-degree relative of those with coeliac disease |
Treatment
[edit]Diet
[edit]At present, the only effective treatment is a lifelong gluten-free diet.[90] Strict adherence to the diet helps the intestines heal, leading to resolution of all symptoms in most cases and, depending on how soon the diet is begun, can also eliminate the heightened risk of osteoporosis and intestinal cancer and in some cases sterility.[91] Compliance to a strict gluten-free diet is difficult for the patient, but evidence has accumulated that a strict gluten-free diet can result in resolution of diarrhoea, weight gain, and normalization of nutrient malabsorption, with normalization of biopsies in 6 months to 2 years on a gluten-free diet.[92]
Dietitians advise which foods contain gluten, which foods are safe, and how to eat a balanced diet despite the limitations. In many countries, gluten-free products are available on prescription and may be reimbursed by health insurance plans. Gluten-free products are usually more expensive and harder to find than common gluten-containing foods.[93] Since ready-made products often contain traces of gluten, some coeliacs may find it necessary to cook from scratch.[94]
The term "gluten-free" is generally used to indicate a supposed harmless level of gluten rather than a complete absence.[95] The exact level at which gluten is harmless is uncertain and controversial. A recent systematic review tentatively concluded that consumption of less than 10 mg of gluten per day is unlikely to cause histological abnormalities, although it noted that few reliable studies had been done.[95] Regulation of the label "gluten-free" varies. In the European Union, the European Commission issued regulations in 2009 limiting the use of "gluten-free" labels for food products to those with less than 20 mg/kg of gluten, and "very low gluten" labels for those with less than 100 mg/kg.[96] In the United States, the FDA issued regulations in 2013 limiting the use of "gluten-free" labels for food products to those with less than 20 ppm[clarification needed]of gluten.[97][98][99] The international Codex Alimentarius standard allows for 20 ppm of gluten in so-called "gluten-free" foods.[100]
A gluten-free diet improves healthcare-related quality of life, and strict adherence to the diet gives more benefit than incomplete adherence. Nevertheless, a gluten-free diet does not completely normalise the quality of life.[101]
Vaccination
[edit]Even though it is unclear if coeliac patients have a generally increased risk of infectious diseases, they should generally be encouraged to receive all common vaccines against vaccine preventable diseases (VPDs) as the general population. Moreover, some pathogens could be harmful to coeliac patients. According to the European Society for the Study of Coeliac Disease (ESsCD), coeliac disease can be associated with hyposplenism or functional asplenia, which could result in impaired immunity to encapsulated bacteria, with an increased risk of such infections. So patients who are known to be hyposplenic should be offered at least the pneumococcal vaccine.[102] However, the ESsCD states that it is not clear whether vaccination with the conjugated vaccine is preferable in this setting and whether additional vaccination against Haemophilus, meningococcus, and influenza should be considered if not previously given.[102]
Refractory disease
[edit]Less than 1% of affected people have refractory disease, which means that they have persistent villous atrophy on a gluten-free diet despite the lack of gluten exposure for more than 12 months.[72] Nevertheless, inadvertent exposure to gluten is the main cause of persistent villous atrophy, and must be ruled out before a diagnosis of refractory disease is made.[75] People with poor basic education and understanding of gluten-free diet often believe that they are strictly following the diet, but are making regular errors.[12][85][103][obsolete source] Also, a lack of symptoms is not a reliable indicator of intestinal recuperation.[85] If alternative causes of villous atrophy have been eliminated, steroids or immunosuppressants (such as azathioprine) may be considered.[104]
Refractory coeliac disease should not be confused with the persistence of symptoms despite gluten withdrawal[85] caused by transient conditions derived from the intestinal damage,[82][83][86] which generally revert or improve several months after starting a gluten-free diet,[87][88] such as small intestinal bacterial overgrowth, lactose intolerance, fructose,[81] sucrose,[82] and sorbitol[83] malabsorption, exocrine pancreatic insufficiency,[84][85] and microscopic colitis[85] among others.
Refractory coeliac disease can be divided into types I and II. A 2023 study compared patients with type I and type II. Refractory coeliac disease type I more frequently exhibits diarrhoea, anaemia, hypoalbuminemia, parenteral nutrition need, ulcerative jejuno-ileitis, and extended small intestinal atrophy. Among patients with refractory coeliac disease type II, it is more common for lymphoma to develop. Among these patients, atrophy extension was the only parameter correlated with hypoalbuminemia and mortality.[non-primary source needed][105]
Epidemiology
[edit]In most countries, between 1 in 50 and 1 in 200 people have coeliac disease.[106] Rates vary in different regions of the world; coeliac disease is less common in places where gluten-containing crops are rarely eaten, and in parts of east Asia and sub-Saharan Africa where populations rarely carry the HLA-DQ genes that predispose to the disease.[106] The risk of developing coeliac disease is higher in those who have a first degree relative with the disease, a less dramatic increase in risk is also seen second degree relatives.[107]
Diagnoses of coeliac disease have increased dramatically in recent decades due to increased awareness of the disease and availability of blood testing. However, the disease is still thought to be underdiagnosed, with an estimated 70% of people with coeliac undiagnosed and untreated. Undiagnosed cases are more common in poorer areas, and in countries which do not regularly test at-risk people.[106]
While coeliac disease can arise at any age, most people develop the disease before age 10.[108] Roughly 20 percent of individuals with coeliac disease are diagnosed after 60 years of age.[109] Coeliac disease is slightly more common in women than in men; though some of that may be due to differences in diagnostic practice – men with gastrointestinal symptoms are less likely to receive a biopsy than women.[108] Other populations at increased risk for coeliac disease, include individuals with Down and Turner syndromes, type 1 diabetes, and autoimmune thyroid disease, including both hyperthyroidism (overactive thyroid) and hypothyroidism (underactive thyroid).[79]
History
[edit]The term coeliac comes from Greek κοιλιακός (koiliakós) 'abdominal' and was introduced in the 19th century in a translation of what is generally regarded as an Ancient Greek description of the disease by Aretaeus of Cappadocia.[110][111]
Humans first started to cultivate grains in the Neolithic period (beginning about 9500 BCE) in the Fertile Crescent in Western Asia, and, likely, coeliac disease did not occur before this time.[112] Aretaeus of Cappadocia, living in the second century in the same area, recorded a malabsorptive syndrome with chronic diarrhoea, causing a debilitation of the whole body.[110]
A 15th-century medical prescription from Mamluk Cairo, attributed to Shams al-Din ibn al-'Afif, the personal physician to Sultan Barsbay and director of the Qalawun complex hospital, describes a treatment for symptoms consistent with coeliac disease. Found in Fustat and now held in the Museum of Islamic Art in Cairo, the remedy combines herbs and plant waters for patients intolerant to wheat.[113]
Aretaeus of Cappadocia's "Cœliac Affection" gained the attention of Western medicine when Francis Adams presented a translation of Aretaeus's work at the Sydenham Society in 1856. The patient described in Aretaeus' work had stomach pain and was atrophied, pale, feeble, and incapable of work. The diarrhoea manifested as loose stools that were white, malodorous, and flatulent, and the disease was intractable and liable to periodic return. The problem, Aretaeus believed, was a lack of heat in the stomach necessary to digest the food and a reduced ability to distribute the digestive products throughout the body, this incomplete digestion resulting in diarrhoea. He regarded this as an affliction of the old and more commonly affecting women, explicitly excluding children. The cause, according to Aretaeus, was sometimes either another chronic disease or even consuming "a copious draught of cold water."[110][111]
The paediatrician Samuel Gee gave the first modern-day description of the condition in children in a lecture at the Hospital for Sick Children, Great Ormond Street, London, in 1887. Gee acknowledged earlier descriptions and terms for the disease and adopted the same term as Aretaeus (coeliac disease). He perceptively stated: "If the patient can be cured at all, it must be by means of diet." Gee recognised that milk intolerance is a problem with coeliac children and that highly starched foods should be avoided. However, he forbade rice, sago, fruit, and vegetables, which all would have been safe to eat, and he recommended raw meat as well as thin slices of toasted bread. Gee highlighted particular success with a child "who was fed upon a quart of the best Dutch mussels daily." However, the child could not bear this diet for more than one season.[111][114]
Christian Archibald Herter, an American physician, wrote a book in 1908 on children with coeliac disease, which he called "intestinal infantilism". He noted their growth was retarded and that fat was better tolerated than carbohydrate. The eponym Gee-Herter disease was sometimes used to acknowledge both contributions.[115][116] Sidney V. Haas, an American paediatrician, reported positive effects of a diet of bananas in 1924.[117] This diet remained in vogue until the actual cause of coeliac disease was determined.[111]
While a role for carbohydrates had been suspected, the link with wheat was not made until the 1940s by the Dutch paediatrician Willem Karel Dicke.[118] It is likely that clinical improvement of his patients during the Dutch famine of 1944–1945 (during which flour was scarce) may have contributed to his discovery.[119] Dicke noticed that the shortage of bread led to a significant drop in the death rate among children affected by coeliac disease from greater than 35% to essentially zero. He also reported that once wheat was again available after the conflict, the mortality rate soared to previous levels.[120] The link with the gluten component of wheat was made in 1952 by a team from Birmingham, England.[121] Villous atrophy was described by British physician John W. Paulley in 1954 on samples taken at surgery.[122] This paved the way for biopsy samples taken by endoscopy.[111]
Throughout the 1960s, other features of coeliac disease were elucidated. Its hereditary character was recognised in 1965.[123] In 1966, dermatitis herpetiformis was linked to gluten sensitivity.[111][124]
Society and culture
[edit]May has been designated as "Coeliac Awareness Month" by several coeliac organisations.[125][126]
Christian churches and the Eucharist
[edit]Speaking generally, the various denominations of Christians celebrate a Eucharist in which a wafer or small piece of sacramental bread from wheat bread is blessed and then eaten. A typical wafer weighs about half a gram.[127] Small communion wafers typically contain 2-5 mg of gliadin if they are not a gluten-free variety,[128] and many people with coeliac disease report altering their religious practices because of coeliac symptoms caused by these wafers.[129]
Many Christian churches offer their communicants gluten-free alternatives, usually in the form of a rice-based cracker or gluten-free bread. These include the United Methodist, Christian Reformed, Episcopal, Anglican and Lutheran Churches. Catholics may receive from the chalice alone, or ask for gluten-reduced hosts; gluten-free ones however are not considered still to be wheat bread, and hence are invalid matter.[130]
Roman Catholic position
[edit]Roman Catholic doctrine states that for a valid Eucharist, the bread to be used at Mass must be made from wheat. Low-gluten hosts meet all of the Catholic Church's requirements, but they are not entirely gluten-free. Requests to use rice wafers have been denied.[131] In 2003, the Congregation for the Doctrine of the Faith stated, "Given the centrality of the celebration of the Eucharist in the life of a priest, one must proceed with great caution before admitting to Holy Orders those candidates unable to ingest gluten or alcohol without serious harm."[132]
By 2004, extremely low-gluten Church-approved hosts had become available in the United States, Italy and Australia.[133] As of 2017, the Vatican still outlawed the use of gluten-free bread for Holy Communion.[134]
Passover
[edit]The Jewish festival of Pesach (Passover) may present problems with its obligation to eat Matzah, which is unleavened bread made in a strictly controlled manner from wheat, barley, spelt, oats, or rye. In addition, many other grains that are normally used as substitutes for people with gluten sensitivity, including rice, are avoided altogether on Passover by Ashkenazi Jews. Many kosher-for-Passover products avoid grains altogether and are therefore gluten-free. Potato starch is the primary starch used to replace the grains.[135]
Spelling
[edit]"Coeliac disease" is the preferred spelling in Commonwealth English, while "celiac disease" is typically used in North American English.[136][137]
Research directions
[edit]The search for environmental factors that could be responsible for genetically susceptible people becoming intolerant to gluten has resulted in increasing research activity looking at gastrointestinal infections.[138] Research published in April 2017 suggests that an often-symptomless infection by a common strain of reovirus can increase sensitivity to foods such as gluten.[139]
Various treatment approaches are being studied, including some that would reduce the need for dieting. All are still under development and are not expected to be available to the general public for a while.[8][140][141] Three main approaches have been proposed: gluten detoxification, modulation of the intestinal permeability, and modulation of the immune response.[142]
Alternatively, gluten exposure can be minimised by the ingestion of a combination of enzymes (prolyl endopeptidase and a barley glutamine-specific cysteine endopeptidase (EP-B2)) that degrade the putative 33-mer peptide in the duodenum.[8] Latiglutenase (IMGX003) is a biotherapeutic digestive enzyme therapy currently being trialled that aims to degrade gluten proteins and aid gluten digestion. It was shown to mitigate intestinal mucosal damage and reduce the severity and frequency of symptoms in phase 2 clinical trials[143] and is scheduled for phase 3 clinical trials.[144]
Other potential approaches to pharmacotherapy include the inhibition of zonulin, an endogenous signalling protein linked to increased permeability of the bowel wall and hence increased presentation of gliadin to the immune system.[145] Other modifiers of other well-understood steps in the pathogenesis of coeliac disease, such as the action of HLA-DQ2 or tissue transglutaminase and the MICA/NKG2D interaction that may be involved in the killing of enterocytes.[8][clarification needed]
Attempts to modulate the immune response concerning coeliac disease are mostly still in phase I of clinical testing; one agent (CCX282-B) has been evaluated in a phase II clinical trial based on small-intestinal biopsies taken from people with coeliac disease before and after gluten exposure.[142][needs update]
References
[edit]- ^ a b Lindfors K, Ciacci C, Kurppa K, Lundin KE, Makharia GK, Mearin ML, et al. (January 2019). "Coeliac disease". Nature Reviews. Disease Primers. 5 (1) 3. doi:10.1038/s41572-018-0054-z. PMID 30631077.
- ^ Matthias T, Pfeiffer S, Selmi C, Eric Gershwin M (April 2010). "Diagnostic challenges in celiac disease and the role of the tissue transglutaminase-neo-epitope". Clin Rev Allergy Immunol (Review). 38 (2–3): 298–301. doi:10.1007/s12016-009-8160-z. PMID 19629760.
- ^ Lewis NR, Scott BB (July 2006). "Systematic review: the use of serology to exclude or diagnose coeliac disease (a comparison of the endomysial and tissue transglutaminase antibody tests)". Alimentary Pharmacology & Therapeutics. 24 (1): 47–54. doi:10.1111/j.1365-2036.2006.02967.x. PMID 16803602.
- ^ Rostom A, Murray JA, Kagnoff MF (December 2006). "American Gastroenterological Association (AGA) Institute technical review on the diagnosis and management of celiac disease". Gastroenterology (Review). 131 (6): 1981–2002. doi:10.1053/j.gastro.2006.10.004. PMID 17087937.
- ^ Molina-Infante J, Santolaria S, Sanders DS, Fernández-Bañares F (May 2015). "Systematic review: noncoeliac gluten sensitivity". Alimentary Pharmacology & Therapeutics (Review). 41 (9): 807–820. doi:10.1111/apt.13155. PMID 25753138.
Furthermore, seronegativity is more common in coeliac disease patients without villous atrophy (Marsh 1-2 lesions), but these 'minor' forms of coeliac disease may have similar clinical manifestations to those with villous atrophy and may show similar clinical–histological remission with reversal of haematological or biochemical disturbances on a gluten-free diet (GFD).
- ^ a b Cichewicz AB, Mearns ES, Taylor A, Boulanger T, Gerber M, Leffler DA, et al. (August 2019). "Diagnosis and Treatment Patterns in Celiac Disease". Digestive Diseases and Sciences (Review). 64 (8): 2095–2106. doi:10.1007/s10620-019-05528-3. PMID 30820708.
- ^ Ludvigsson JF, Card T, Ciclitira PJ, Swift GL, Nasr I, Sanders DS, et al. (April 2015). "Support for patients with celiac disease: A literature review". United European Gastroenterology Journal (Review). 3 (2): 146–159. doi:10.1177/2050640614562599. PMC 4406900. PMID 25922674.
- ^ a b c d e van Heel DA, West J (July 2006). "Recent advances in coeliac disease". Gut (Review). 55 (7): 1037–1046. doi:10.1136/gut.2005.075119. PMC 1856316. PMID 16766754.
- ^ a b Bibbins-Domingo K, Grossman DC, Curry SJ, Barry MJ, Davidson KW, Doubeni CA, et al. (March 2017). "Screening for Celiac Disease: US Preventive Services Task Force Recommendation Statement". JAMA. 317 (12): 1252–1257. doi:10.1001/jama.2017.1462. PMID 28350936.
- ^ Burkhardt JG, Chapa-Rodriguez A, Bahna SL (July 2018). "Gluten sensitivities and the allergist: Threshing the grain from the husks". Allergy. 73 (7): 1359–1368. doi:10.1111/all.13354. PMID 29131356.
- ^ Costantino A, Aversano GM, Lasagni G, Smania V, Doneda L, Vecchi M, et al. (2022). "Diagnostic management of patients reporting symptoms after wheat ingestion". Frontiers in Nutrition. 9 1007007. doi:10.3389/fnut.2022.1007007. PMC 9582535. PMID 36276818.
- ^ a b c See JA, Kaukinen K, Makharia GK, Gibson PR, Murray JA (October 2015). "Practical insights into gluten-free diets". Nature Reviews. Gastroenterology & Hepatology (Review). 12 (10): 580–591. doi:10.1038/nrgastro.2015.156. PMID 26392070.
A lack of symptoms and/or negative serological markers are not reliable indicators of mucosal response to the diet. Furthermore, up to 30% of patients continue to have gastrointestinal symptoms despite a strict GFD.122,124 If adherence is questioned, a structured interview by a qualified dietitian can help to identify both intentional and inadvertent sources of gluten.
- ^ Lebwohl B, Ludvigsson JF, Green PH (October 2015). "Celiac disease and non-celiac gluten sensitivity". BMJ (Review). 351 h4347. doi:10.1136/bmj.h4347. PMC 4596973. PMID 26438584.
Celiac disease occurs in about 1% of the population worldwide, although most people with the condition are undiagnosed. It can cause a wide variety of symptoms, both intestinal and extra-intestinal, because it is a systemic autoimmune disease that is triggered by dietary gluten. Patients with coeliac disease are at increased risk of cancer, including a twofold to fourfold increased risk of non-Hodgkin lymphoma and a more than 30-fold increased risk of small intestinal adenocarcinoma, and they have a 1.4-fold increased risk of death.
- ^ Fasano A, Catassi C (December 2012). "Clinical practice. Celiac disease". The New England Journal of Medicine (Review). 367 (25): 2419–2426. doi:10.1056/NEJMcp1113994. PMID 23252527.
- ^ "Celiac disease". World Gastroenterology Organisation Global Guidelines. July 2016. Archived from the original on 17 March 2017. Retrieved 23 April 2017.
- ^ a b Lionetti E, Gatti S, Pulvirenti A, Catassi C (June 2015). "Celiac disease from a global perspective". Best Practice & Research. Clinical Gastroenterology (Review). 29 (3): 365–379. doi:10.1016/j.bpg.2015.05.004. PMID 26060103.
- ^ Hischenhuber C, Crevel R, Jarry B, Mäki M, Moneret-Vautrin DA, Romano A, et al. (March 2006). "Review article: safe amounts of gluten for patients with wheat allergy or coeliac disease". Alimentary Pharmacology & Therapeutics. 23 (5): 559–575. doi:10.1111/j.1365-2036.2006.02768.x. PMID 16480395.
- ^ a b Varma S, Krishnareddy S (2022). "Uncomplicated Celiac Disease". Refractory Celiac Disease. Cham: Springer International Publishing. pp. 5–19. doi:10.1007/978-3-030-90142-4_2. ISBN 978-3-030-90141-7. Retrieved 17 August 2025.
- ^ a b c d e Catassi C, Verdu EF, Bai JC, Lionetti E (2022). "Coeliac disease". The Lancet. 399 (10344): 2413–2426. doi:10.1016/S0140-6736(22)00794-2. PMID 35691302. Retrieved 22 August 2025.
- ^ a b c d e Tarar ZI, Zafar MU, Farooq U, Basar O, Tahan V, Daglilar E (2021). "The Progression of Celiac Disease, Diagnostic Modalities, and Treatment Options". Journal of Investigative Medicine High Impact Case Reports. 9 23247096211053702. doi:10.1177/23247096211053702. ISSN 2324-7096. PMC 8767653. PMID 34693776.
- ^ a b c d Lebwohl B, Rubio-Tapia A (2021). "Epidemiology, Presentation, and Diagnosis of Celiac Disease". Gastroenterology. 160 (1): 63–75. doi:10.1053/j.gastro.2020.06.098. PMID 32950520. Retrieved 20 August 2025.
- ^ a b Kvamme JM, Sørbye S, Florholmen J, Halstensen TS (25 July 2022). "Population-based screening for celiac disease reveals that the majority of patients are undiagnosed and improve on a gluten-free diet" (PDF). Scientific Reports. 12 (1) 12647. Bibcode:2022NatSR..1212647K. doi:10.1038/s41598-022-16705-2. ISSN 2045-2322. PMC 9314380. PMID 35879335. Retrieved 20 August 2025.
- ^ a b de Villasante GC (2022). "Classical and Non-classical Forms of CD in Paediatrics". Advances in Celiac Disease. Cham: Springer International Publishing. pp. 23–33. doi:10.1007/978-3-030-82401-3_3. ISBN 978-3-030-82400-6. Retrieved 17 August 2025.
- ^ a b c d e Ludvigsson JF, Yao J, Lebwohl B, Green PH, Yuan S, Leffler DA (28 January 2025). "Coeliac disease: complications and comorbidities". Nature Reviews Gastroenterology & Hepatology. 22 (4): 252–264. doi:10.1038/s41575-024-01032-w. ISSN 1759-5045. PMID 39875649. Retrieved 20 August 2025.
- ^ Lenti MV, Hammer HF, Tacheci I, Burgos R, Schneider S, Foteini A, et al. (2025). "European Consensus on Malabsorption—UEG & SIGE, LGA, SPG, SRGH, CGS, ESPCG, EAGEN, ESPEN, and ESPGHAN: Part 2: Screening, Special Populations, Nutritional Goals, Supportive Care, Primary Care Perspective". United European Gastroenterology Journal. 13 (5): 773–797. doi:10.1002/ueg2.70011. ISSN 2050-6406. PMC 12188380. PMID 40088199.
- ^ Laurikka P, Kivelä L, Kurppa K, Kaukinen K (July 2022). "Review article: Systemic consequences of coeliac disease". Alimentary Pharmacology & Therapeutics. 56 (S1): S64 – S72. doi:10.1111/apt.16912. ISSN 0269-2813. PMC 9543231. PMID 35815828.
- ^ a b c d e f g h Lazzano P, Fracas E, Nandi N, Scaramella L, Elli L (2024). "Extraintestinal complications of celiac disease: treatment considerations". Expert Review of Gastroenterology & Hepatology. 18 (12): 761–777. doi:10.1080/17474124.2024.2443053. ISSN 1747-4124. PMID 39673511. Retrieved 20 August 2025.
- ^ a b c d e f Therrien A, Kelly CP, Silvester JA (2020). "Celiac Disease: Extraintestinal Manifestations and Associated Conditions". Journal of Clinical Gastroenterology. 54 (1): 8–21. doi:10.1097/MCG.0000000000001267. ISSN 0192-0790. PMC 6895422. PMID 31513026.
- ^ Nuermaimaiti K, Li T, Li N, Shi T, Liu W, Abulaiti P, et al. (3 August 2025). "Vitamin and trace elements imbalance are very common in adult patients with newly diagnosed Celiac disease". Scientific Reports. 15 (1) 28315. Bibcode:2025NatSR..1528315N. doi:10.1038/s41598-025-12631-1. ISSN 2045-2322. PMC 12319082. PMID 40754594.
- ^ Mosca C, Thorsteinsdottir F, Abrahamsen B, Rumessen JJ, Händel MN (3 January 2022). "Newly Diagnosed Celiac Disease and Bone Health in Young Adults: A Systematic Literature Review" (PDF). Calcified Tissue International. 110 (6): 641–648. doi:10.1007/s00223-021-00938-w. ISSN 1432-0827. PMC 8721639. PMID 34978602. Retrieved 20 August 2025.
- ^ Poddighe D, Capittini C (13 December 2021). "The Role of HLA in the Association between IgA Deficiency and Celiac Disease". Disease Markers. 2021: 1–8. doi:10.1155/2021/8632861. ISSN 1875-8630. PMC 8856801. PMID 35186163.
- ^ Lebwohl B, Green PH, Emilsson L, Mårild K, Söderling J, Roelstraete B, et al. (2022). "Cancer Risk in 47,241 Individuals With Celiac Disease: A Nationwide Cohort Study". Clinical Gastroenterology and Hepatology. 20 (2): e111 – e131. doi:10.1016/j.cgh.2021.05.034. PMID 34033925. Retrieved 20 August 2025.
- ^ Chornenkyy Y, Peric M, Flores DM, Ono Y, Shinagare SA, Dannheim K, et al. (2025). "Ulcerative Jejunitis in Celiac Disease: A 30-Year US Experience". American Journal of Gastroenterology. 120 (6): 1353–1366. doi:10.14309/ajg.0000000000003170. ISSN 0002-9270. PMID 39471499. Retrieved 20 August 2025.
- ^ a b Ahmadzadeh A, Rezaei-Tavirani M (6 May 2025). "Pathogenesis and genetics of celiac disease; a systematic review". Egyptian Journal of Medical Human Genetics. 26 (1) 85. doi:10.1186/s43042-025-00713-8. ISSN 2090-2441.
- ^ a b c d e f Calado J, Verdelho Machado M (2022). "Celiac Disease Revisited". GE - Portuguese Journal of Gastroenterology. 29 (2): 111–124. doi:10.1159/000514716. ISSN 2341-4545. PMC 8995660. PMID 35497669. Retrieved 20 August 2025.
- ^ Biesiekierski JR (March 2017). "What is gluten?". Journal of Gastroenterology and Hepatology. 32 (Suppl 1): 78–81. doi:10.1111/jgh.13703. PMID 28244676.
Similar proteins to the gliadin found in wheat exist as secalin in rye, hordein in barley, and avenins in oats and are collectively referred to as "gluten." Derivatives of these grains, such as triticale and malt, and other ancient wheat varieties, such as spelt and kamut, also contain gluten. The gluten found in all of these grains has been identified as the component capable of triggering the immune-mediated disorder, coeliac disease.
- ^ McDermid JM, Almond MA, Roberts KM, Germer EM, Geller MG, Taylor TA, et al. (2023). "Celiac Disease: An Academy of Nutrition and Dietetics Evidence-Based Nutrition Practice Guideline". Journal of the Academy of Nutrition and Dietetics. 123 (12): 1793–1807.e4. doi:10.1016/j.jand.2023.07.018. PMID 37499866. Retrieved 20 August 2025.
- ^ Kosová K, Leišová-Svobodová L, Dvořáček V (2020). "Oats as a Safe Alternative to Triticeae Cereals for People Suffering from Celiac Disease? A Review". Plant Foods for Human Nutrition. 75 (2): 131–141. Bibcode:2020PFHN...75..131K. doi:10.1007/s11130-020-00800-8. ISSN 0921-9668. PMID 32133597. Retrieved 20 August 2025.
- ^ Lee AR, Dennis M, Lebovits J, Welstead L, Verma R, Therrien A, et al. (2025). "Dietary assessments in individuals living with coeliac disease: key considerations". Journal of Human Nutrition and Dietetics. 38 (1) e13380. doi:10.1111/jhn.13380. ISSN 0952-3871. PMC 11589401. PMID 39501424.
- ^ Raiteri A, Granito A, Giamperoli A, Catenaro T, Negrini G, Tovoli F (7 January 2022). "Current guidelines for the management of celiac disease: A systematic review with comparative analysis". World Journal of Gastroenterology. 28 (1): 154–176. doi:10.3748/wjg.v28.i1.154. ISSN 1007-9327. PMC 8793016. PMID 35125825.
- ^ Demirkesen I, Ozkaya B (25 January 2022). "Recent strategies for tackling the problems in gluten-free diet and products". Critical Reviews in Food Science and Nutrition. 62 (3): 571–597. doi:10.1080/10408398.2020.1823814. ISSN 1040-8398. PMID 32981341. Retrieved 21 August 2025.
- ^ Luque V, Crespo-Escobar P, Hård af Segerstad EM, Koltai T, Norsa L, Roman E, et al. (2024). "Gluten-free diet for pediatric patients with coeliac disease: A position paper from the ESPGHAN gastroenterology committee, special interest group in coeliac disease". Journal of Pediatric Gastroenterology and Nutrition. 78 (4): 973–995. doi:10.1002/jpn3.12079. ISSN 0277-2116. PMID 38291739.
- ^ a b c d e f g Leffler DA, Dennis M, Lebwohl B (15 April 2022). "Celiac disease". Yamada's Textbook of Gastroenterology. Wiley. pp. 1122–1136. doi:10.1002/9781119600206.ch56. ISBN 978-1-119-60016-9. Retrieved 21 August 2025.
- ^ Szajewska H, Shamir R, Stróżyk A, Chmielewska A, Zalewski BM, Auricchio R, et al. (2023). "Systematic review: early feeding practices and the risk of coeliac disease. A 2022 update and revision". Alimentary Pharmacology & Therapeutics. 57 (1): 8–22. doi:10.1111/apt.17290. hdl:1887/3576015. ISSN 0269-2813. PMID 36411726. Retrieved 21 August 2025.
- ^ Mearin ML (2022). "Celiac Disease Prevention". Advances in Celiac Disease. Cham: Springer International Publishing. pp. 153–159. doi:10.1007/978-3-030-82401-3_11. ISBN 978-3-030-82400-6. Retrieved 21 August 2025.
- ^ a b Gnodi E, Meneveri R, Barisani D (28 January 2022). "Celiac disease: From genetics to epigenetics". World Journal of Gastroenterology. 28 (4): 449–463. doi:10.3748/wjg.v28.i4.449. ISSN 1007-9327. PMC 8790554. PMID 35125829.
- ^ a b Galipeau HJ, Hinterleitner R, Leonard MM, Caminero A (2024). "Non-Host Factors Influencing Onset and Severity of Celiac Disease". Gastroenterology. 167 (1): 34–50. doi:10.1053/j.gastro.2024.01.030. PMC 11653303. PMID 38286392.
- ^ a b c Gaba K, Malhotra P, Kumar A, Suneja P, Dang AS (2024). "Understanding the Genetic Basis of Celiac Disease: A Comprehensive Review". Cell Biochemistry and Biophysics. 82 (3): 1797–1808. doi:10.1007/s12013-024-01371-0. ISSN 1085-9195. PMID 38907939. Retrieved 23 August 2025.
- ^ Kim C, Quarsten H, Bergseng E, Khosla C, Sollid L (2004). "Structural basis for HLA-DQ2-mediated presentation of gluten epitopes in celiac disease". Proc Natl Acad Sci USA. 101 (12): 4175–4179. Bibcode:2004PNAS..101.4175K. doi:10.1073/pnas.0306885101. PMC 384714. PMID 15020763.
- ^ Choung RS, Mills JR, Snyder MR, Murray JA, Gandhi MJ (2020). "Celiac disease risk stratification based on HLA-DQ heterodimer (HLA-DQA1 ~ DQB1) typing in a large cohort of adults with suspected celiac disease". Human Immunology. 81 (2–3): 59–64. doi:10.1016/j.humimm.2020.01.006. PMID 32005535. Retrieved 22 August 2025.
- ^ Apcher S, Vojtesek B, Fahraeus R (2023). "In search of the cell biology for self- versus non-self- recognition". Current Opinion in Immunology. 83 102334. doi:10.1016/j.coi.2023.102334. PMID 37210933.
- ^ Jin H, Arase H (2024). "Neoself Antigens Presented on MHC Class II Molecules in Autoimmune Diseases". Basic Immunology and Its Clinical Application. Advances in Experimental Medicine and Biology. Vol. 1444. Singapore: Springer Nature Singapore. pp. 51–65. doi:10.1007/978-981-99-9781-7_4. ISBN 978-981-99-9780-0. PMID 38467972. Retrieved 7 September 2025.
- ^ a b c Núñez C, Rubio M (2022). "Value and Use of Genetic Test of Celiac Disease". Advances in Celiac Disease. Cham: Springer International Publishing. pp. 99–119. doi:10.1007/978-3-030-82401-3_8. ISBN 978-3-030-82400-6. Retrieved 22 August 2025.
- ^ Siddiqui K, Uqaili AA, Rafiq M, Bhutto MA (19 March 2021). "Human leukocyte antigen (HLA)-DQ2 and -DQ8 haplotypes in celiac, celiac with type 1 diabetic, and celiac suspected pediatric cases". Medicine. 100 (11) e24954. doi:10.1097/MD.0000000000024954. ISSN 0025-7974. PMC 7982179. PMID 33725967.
- ^ Tolone C, Piccirillo M, Dolce P, Alfiero S, Arenella M, Sarnataro M, et al. (2021). "Celiac disease in pediatric patients according to HLA genetic risk classes: a retrospective observational study". Italian Journal of Pediatrics. 47 (1) 107. doi:10.1186/s13052-021-01052-1. ISSN 1824-7288. PMC 8097774. PMID 33952340.
- ^ a b c Pritchard D, Anand A, De'Ath A, Lee H, Rees MT (2024). "UK NEQAS and BSHI guideline: Laboratory testing and clinical interpretation of HLA genotyping results supporting the diagnosis of coeliac disease". International Journal of Immunogenetics. 51 (S1): 3–20. doi:10.1111/iji.12649. ISSN 1744-3121. PMID 38153308. Retrieved 23 August 2025.
- ^ a b Del Pozzo G, Farina F, Picascia S, Laezza M, Vitale S, Gianfrani C (2021). "HLA class II genes in precision-based care of childhood diseases: what we can learn from celiac disease" (PDF). Pediatric Research. 89 (2): 307–312. doi:10.1038/s41390-020-01217-4. ISSN 0031-3998. PMID 33122841. Retrieved 12 September 2025.
- ^ Makharia GK, Singh P, Catassi C, Sanders DS, Leffler D, Ali RA, et al. (2022). "The global burden of coeliac disease: opportunities and challenges". Nature Reviews Gastroenterology & Hepatology. 19 (5): 313–327. doi:10.1038/s41575-021-00552-z. ISSN 1759-5045. PMID 34980921. Retrieved 12 September 2025.
- ^ Espino L, Núñez C (2021). "The HLA complex and coeliac disease". International Review of Cell and Molecular Biology. Vol. 358. Elsevier. pp. 47–83. doi:10.1016/bs.ircmb.2020.09.009. ISBN 978-0-323-85311-8. PMID 33707057. Retrieved 12 September 2025.
- ^ a b c d Arranz E, Garrote JA (2022). "Immunopathogenesis of Celiac Disease". Advances in Celiac Disease. Cham: Springer International Publishing. pp. 35–49. doi:10.1007/978-3-030-82401-3_4. ISBN 978-3-030-82400-6. Retrieved 23 August 2025.
- ^ a b Qiao SW, Bergseng E, Molberg Ø, Xia J, Fleckenstein B, Khosla C, et al. (August 2004). "Antigen presentation to celiac lesion-derived T cells of a 33-mer gliadin peptide naturally formed by gastrointestinal digestion". Journal of Immunology. 173 (3): 1757–1762. doi:10.4049/jimmunol.173.3.1757. PMID 15265905.
- ^ a b Yao Z, Fan Y, Lin L, Kellems RE, Xia Y (1 January 2024). "Tissue transglutaminase: a multifunctional and multisite regulator in health and disease". Physiological Reviews. 104 (1): 281–325. doi:10.1152/physrev.00003.2023. ISSN 0031-9333. PMID 37712623. Retrieved 24 August 2025.
- ^ Amundsen SF, Stamnaes J, du Pré MF, Sollid LM (2022). "Transglutaminase 2 affinity and enzyme-substrate intermediate stability as determining factors for T-cell responses to gluten peptides in celiac disease" (PDF). European Journal of Immunology. 52 (9): 1474–1481. doi:10.1002/eji.202249862. ISSN 0014-2980. PMC 9545004. PMID 35715890. Retrieved 24 August 2025.
- ^ Lexhaller B, Ludwig C, Scherf KA (4 May 2020). "Identification of Isopeptides Between Human Tissue Transglutaminase and Wheat, Rye, and Barley Gluten Peptides" (PDF). Scientific Reports. 10 (1) 7426. Bibcode:2020NatSR..10.7426L. doi:10.1038/s41598-020-64143-9. ISSN 2045-2322. PMC 7198585. PMID 32367038. Retrieved 24 August 2025.
- ^ a b c d Shiha MG, Sanders DS (2025). "What is new in the management of coeliac disease?". European Journal of Internal Medicine. 134: 1–8. doi:10.1016/j.ejim.2025.01.028. PMID 39894725. Retrieved 29 August 2025.
- ^ Dhar J, Samanta J, Sharma M, Kumar S, Sinha SK, Kochhar R (2022). "Impact of delay in diagnosis in patients with celiac disease: A study of 570 patients at a tertiary care center". Indian Journal of Gastroenterology. 41 (1): 30–36. doi:10.1007/s12664-021-01214-3. ISSN 0254-8860. PMID 35064913. Retrieved 29 August 2025.
- ^ Kårhus LL, Hansen S, Rumessen JJ, Linneberg A (28 December 2022). "Diagnostic Delay in Coeliac Disease: A Survey among Danish Patients". Canadian Journal of Gastroenterology and Hepatology. 2022: 1–7. doi:10.1155/2022/5997624. ISSN 2291-2797. PMC 9812619. PMID 36618766.
- ^ a b Tye-Din JA (2024). "Evolution in coeliac disease diagnosis and management". JGH Open. 8 (7) e13107. doi:10.1002/jgh3.13107. ISSN 2397-9070. PMC 11217771. PMID 38957478.
- ^ a b c d e f g Shiha MG, Hadjisavvas N, Sanders DS, Penny HA (30 September 2024). "Optimising the Diagnosis of Adult Coeliac Disease: Current Evidence and Future Directions". British Journal of Hospital Medicine. 85 (9): 1–21. doi:10.12968/hmed.2024.0362. ISSN 1750-8460. PMID 39347683. Retrieved 29 August 2025.
- ^ a b Elli L, Leffler D, Cellier C, Lebwohl B, Ciacci C, Schumann M, et al. (2024). "Guidelines for best practices in monitoring established coeliac disease in adult patients" (PDF). Nature Reviews Gastroenterology & Hepatology. 21 (3): 198–215. doi:10.1038/s41575-023-00872-2. ISSN 1759-5045. PMID 38110546. Retrieved 29 August 2025.
- ^ a b Husby S, Koletzko S, Korponay-Szabó I, Kurppa K, Mearin ML, Ribes-Koninckx C, et al. (2020). "European Society Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for Diagnosing Coeliac Disease 2020". Journal of Pediatric Gastroenterology and Nutrition. 70 (1): 141–156. doi:10.1097/MPG.0000000000002497. hdl:1887/3184859. ISSN 0277-2116. PMID 31568151.
- ^ a b c d e f g Rubio-Tapia A, Hill ID, Semrad C, Kelly CP, Greer KB, Limketkai BN, et al. (2023). "American College of Gastroenterology Guidelines Update: Diagnosis and Management of Celiac Disease". American Journal of Gastroenterology. 118 (1): 59–76. doi:10.14309/ajg.0000000000002075. ISSN 0002-9270. PMID 36602836.
- ^ a b c d Ribes-Koninckx C, Roca M, Donat E (2022). "Value and Use of Serologic Markers of Celiac Disease". Advances in Celiac Disease. Cham: Springer International Publishing. pp. 63–78. doi:10.1007/978-3-030-82401-3_6. ISBN 978-3-030-82400-6. Retrieved 30 August 2025.
- ^ a b c d e Anderson RP (2022). "Review article: Diagnosis of coeliac disease: a perspective on current and future approaches". Alimentary Pharmacology & Therapeutics. 56 (S1): S18 – S37. doi:10.1111/apt.16840. ISSN 0269-2813. PMID 35815826. Retrieved 30 August 2025.
- ^ a b c d e Shiha MG, Chetcuti Zammit S, Elli L, Sanders DS, Sidhu R (2023). "Updates in the diagnosis and management of coeliac disease". Best Practice & Research Clinical Gastroenterology. 64–65 101843. doi:10.1016/j.bpg.2023.101843. PMID 37652646. Retrieved 31 August 2025.
- ^ Di Tola M, Bizzaro N, Gaudio M, Maida C, Villalta D, Alessio MG, et al. (2021). "Diagnosing and Monitoring Celiac Patients with Selective IgA Deficiency: Still an Open Issue". Digestive Diseases and Sciences. 66 (10): 3234–3241. doi:10.1007/s10620-021-07204-x. ISSN 0163-2116. PMID 34383199. Retrieved 31 August 2025.
- ^ a b Yoon SM (2022). "Celiac Disease". Small Intestine Disease. Singapore: Springer Singapore. pp. 265–267. doi:10.1007/978-981-16-7239-2_51. ISBN 978-981-16-7238-5. Retrieved 15 November 2025.
- ^ a b c Vincenzo V, Gloria S, Melissa M, Alessandro C, Rachele DS (2022). "Histopathological Assessment of Celiac Disease". Advances in Celiac Disease. Cham: Springer International Publishing. pp. 79–97. doi:10.1007/978-3-030-82401-3_7. ISBN 978-3-030-82400-6. Retrieved 14 September 2025.
- ^ a b c d Butterworth J, Los L (2024). "Coeliac disease". Medicine. 52 (3): 174–180. doi:10.1016/j.mpmed.2023.12.003. Retrieved 14 September 2025.
- ^ Vivas S, Vaquero L, Rodríguez-Martín L, Caminero A (November 2015). "Age-related differences in celiac disease: Specific characteristics of adult presentation". World Journal of Gastrointestinal Pharmacology and Therapeutics (Review). 6 (4): 207–212. doi:10.4292/wjgpt.v6.i4.207. PMC 4635160. PMID 26558154.
In addition, the presence of intraepithelial lymphocytosis and/or villous atrophy and crypt hyperplasia of small-bowel mucosa, and clinical remission after withdrawal of gluten from the diet, are also used for diagnosis antitransglutaminase antibody (tTGA) titers and the degree of histological lesions inversely correlate with age. Thus, as the age of diagnosis increases, antibody titers decrease, and histological damage is less marked. It is common to find adults without villous atrophy showing only an inflammatory pattern in duodenal mucosa biopsies: Lymphocytic enteritis (Marsh I) or added crypt hyperplasia (Marsh II)
- ^ a b Castillo NE, Theethira TG, Leffler DA (February 2015). "The present and the future in the diagnosis and management of celiac disease". Gastroenterology Report (Review). 3 (1): 3–11. doi:10.1093/gastro/gou065. PMC 4324867. PMID 25326000.
- ^ a b c d e Levy J, Bernstein L, Silber N (December 2014). "Celiac disease: an immune dysregulation syndrome". Current Problems in Pediatric and Adolescent Health Care (Review). 44 (11): 324–327. doi:10.1016/j.cppeds.2014.10.002. PMID 25499458.
Initially, reduced levels of lactase and sucrase activities might necessitate further dietary restrictions until the villi have healed and those sugars are better tolerated.
- ^ a b c d Montalto M, Gallo A, Ojetti V, Gasbarrini A (2013). "Fructose, trehalose and sorbitol malabsorption" (PDF). European Review for Medical and Pharmacological Sciences (Review). 17 (Suppl 2): 26–29. PMID 24443064. Archived (PDF) from the original on 12 April 2016.
- ^ a b Leffler DA, Green PH, Fasano A (October 2015). "Extraintestinal manifestations of coeliac disease". Nature Reviews. Gastroenterology & Hepatology (Review). 12 (10): 561–571. doi:10.1038/nrgastro.2015.131. PMID 26260366.
- ^ a b c d e f g Woodward J (3 August 2016). "Improving outcomes of refractory celiac disease - current and emerging treatment strategies". Clinical and Experimental Gastroenterology (Review). 9: 225–236. doi:10.2147/ceg.s87200. PMC 4976763. PMID 27536154.
- ^ a b c Berni Canani R, Pezzella V, Amoroso A, Cozzolino T, Di Scala C, Passariello A (March 2016). "Diagnosing and Treating Intolerance to Carbohydrates in Children". Nutrients. 8 (3): 157. doi:10.3390/nu8030157. PMC 4808885. PMID 26978392.
- ^ a b c García-Manzanares A, Lucendo AJ (April 2011). "Nutritional and dietary aspects of celiac disease". Nutrition in Clinical Practice (Review). 26 (2): 163–173. doi:10.1177/0884533611399773. PMID 21447770.
- ^ a b c d Green PH, Jabri B (August 2003). "Coeliac disease". Lancet (Review). 362 (9381): 383–391. doi:10.1016/S0140-6736(03)14027-5. PMID 12907013.
- ^ Volta U, Caio G, Tovoli F, De Giorgio R (September 2013). "Non-celiac gluten sensitivity: questions still to be answered despite increasing awareness". Cellular & Molecular Immunology (Review). 10 (5): 383–392. doi:10.1038/cmi.2013.28. PMC 4003198. PMID 23934026.
- ^ Kupper C (April 2005). "Dietary guidelines and implementation for celiac disease". Gastroenterology. 128 (4 Suppl 1): S121 – S127. doi:10.1053/j.gastro.2005.02.024. PMID 15825119.
- ^ Treem WR (2004). "Emerging concepts in celiac disease". Curr Opin Pediatr. 16 (5): 552–559. doi:10.1097/01.mop.0000142347.74135.73. PMID 15367850.
- ^ Freeman HJ (2017). "Dietary compliance in celiac disease". World J Gastroenterol. 23 (15): 2635–2639. doi:10.3748/wjg.v23.i15.2635. PMID 15367850.
- ^ Lee AR, Ng DL, Zivin J, Green PH (2007). "Economic burden of a gluten-free diet" (PDF). J Hum Nutr Diet. 20 (5): 423–430. CiteSeerX 10.1.1.662.8399. doi:10.1111/j.1365-277X.2007.00763.x. PMID 17845376. Archived from the original (PDF) on 24 June 2015. Retrieved 9 February 2018.
- ^ Troncone R, Ivarsson A, Szajewska H, Mearin ML (2008). "Review article: future research on coeliac disease – a position report from the European multistakeholder platform on coeliac disease (CDEUSSA)". Aliment. Pharmacol. Ther. 27 (11): 1030–1043. doi:10.1111/j.1365-2036.2008.03668.x. PMID 18315588.
- ^ a b Akobeng AK, Thomas AG (June 2008). "Systematic review: tolerable amount of gluten for people with coeliac disease". Aliment. Pharmacol. Ther. 27 (11): 1044–1052. doi:10.1111/j.1365-2036.2008.03669.x. PMID 18315587.
- ^ "Gluten-free food". Directorate-General for Health and Consumers. Archived from the original on 23 July 2015. Retrieved 25 July 2015.
- ^ "What is Gluten-Free? FDA Has an Answer". Food and Drug Administration. 2 August 2013. Archived from the original on 4 August 2013. Retrieved 2 August 2013.
As one of the criteria for using the claim 'gluten-free,' FDA is setting a gluten limit of less than 20 ppm (parts per million) in foods that carry this label. This is the lowest level that can be consistently detected in foods using valid scientific analytical tools. Also, most people with celiac disease can tolerate foods with very small amounts of gluten. This level is consistent with those set by other countries and international bodies that set food safety standards.
- ^ Section 206 of the Food Allergen Labeling and Consumer Protection Act of 2004, Title II of Pub. L. 108–282 (text) (PDF), 118 Stat. 891 , enacted August 2, 2004
- ^ 78 FR 47154 (5 August 2013). Codified at 21 CFR 101.91.
- ^ Codex Committee on Nutrition and Foods for Special Dietary Uses (CCNFSDU). Standard for foods for special dietary use for persons intolerant to gluten (PDF) (Report) (Adopted in 1979. Amended in 1983 and 2015. Revised in 2008 ed.). Food and Agriculture Organization (FAO) and World Health Organization (WHO). Codex Alimentarius (CXS 118-1979).
- ^ Burger JP, de Brouwer B, IntHout J, Wahab PJ, Tummers M, Drenth JP (April 2017). "Systematic review with meta-analysis: Dietary adherence influences normalization of health-related quality of life in coeliac disease". Clinical Nutrition. 36 (2): 399–406. doi:10.1016/j.clnu.2016.04.021. PMID 27179800.
- ^ a b Al-Toma A, Volta U, Auricchio R, Castillejo G, Sanders DS, Cellier C, et al. (June 2019). "European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders". United European Gastroenterology Journal. 7 (5): 583–613. doi:10.1177/2050640619844125. PMC 6545713. PMID 31210940.
- ^ Mulder CJ, van Wanrooij RL, Bakker SF, Wierdsma N, Bouma G (2013). "Gluten-free diet in gluten-related disorders". Dig. Dis. (Review). 31 (1): 57–62. doi:10.1159/000347180. PMID 23797124.
- ^ "American Gastroenterological Association medical position statement: Celiac Sprue". Gastroenterology. 120 (6): 1522–1525. May 2001. doi:10.1053/gast.2001.24055. PMID 11313323.
- ^ Elli L, et al. (February 2023). "Clinical features of type 1 and 2 refractory celiac disease: Results from a large cohort over a decade". Digestive and Liver Disease. 55 (2): 235–242. doi:10.1016/j.dld.2022.08.022. PMID 36096991.
- ^ a b c Catassi C, Verdu EF, Boi C, Lionetti E (2022). "Coeliac disease". Lancet. 399 (10344): 2413–2426. doi:10.1016/S0140-6736(22)00794-2. PMID 35691302.
- ^ Rajput MS, Chauhan A, Makharia GK (2022). "Epidemiology of Celiac Disease". Advances in Celiac Disease. Cham: Springer International Publishing. p. 7–22. doi:10.1007/978-3-030-82401-3_2. ISBN 978-3-030-82400-6. Retrieved 3 November 2025.
- ^ a b Lebwohl B, Rubio-Tapia A (January 2021). "Epidemiology, Presentation, and Diagnosis of Celiac Disease". Gastroenterology. 160 (1): 63–75. doi:10.1053/j.gastro.2020.06.098. PMID 32950520.
- ^ Ching CK, Lebwohl B (5 August 2022). "Celiac Disease in the Elderly". Current Treatment Options in Gastroenterology. 20 (3): 238–249. doi:10.1007/s11938-022-00397-8. ISSN 1534-309X. PMC 9937540. PMID 36818495.
- ^ a b c Aretaeus, the Cappadocian (1856). "On The Cœliac Affection". The extant works of Aretaeus, The Cappadocian. Translated by Francis Adams. London: Sydenham Society. pp. 350–351. Retrieved 12 December 2009.
- ^ a b c d e f Losowsky MS (2008). "A history of coeliac disease". Digestive Diseases. 26 (2): 112–120. doi:10.1159/000116768. PMID 18431060.
- ^ Freeman HJ (15 January 2015). "Celiac Disease: A Disorder Emerging from Antiquity, Its Evolving Classification and Risk, and Potential New Treatment Paradigms". Gut and Liver. 9 (1): 28–37. doi:10.5009/gnl14288. ISSN 1976-2283. PMC 4282854. PMID 25547088.
- ^ Sayour S. "Medical prescription in Discover Islamic Art, Museum With No Frontiers, 2025". islamicart.museumwnf.org. Retrieved 21 April 2025.
- ^ Gee SJ (1888). "On the coeliac affection". St Bartholomew's Hospital Report. 24: 17–20. Archived from the original on 26 September 2007. Retrieved 20 March 2007.
- ^ Herter CA (1908). On infantilism from chronic intestinal infection; characterized by the overgrowth and persistence of flora in the nursing period. New York: Macmillan & Co. as cited by WhoNamedIt
- ^ Enersen OD. "Christian Archibald Herter". Who Named It?. Archived from the original on 31 December 2006. Retrieved 20 March 2007.
- ^ Haas SV (1924). "The value of the banana in the treatment of coeliac disease". Am J Dis Child. 24 (4): 421–437. doi:10.1001/archpedi.1924.04120220017004.
- ^ van Berge-Henegouwen GP, Mulder CJ (1993). "Pioneer in the gluten free diet: Willem-Karel Dicke 1905–1962, over 50 years of gluten free diet". Gut. 34 (11): 1473–1475. doi:10.1136/gut.34.11.1473. PMC 1374403. PMID 8244125.
- ^ Dicke WK (1950). Coeliakie: een onderzoek naar de nadelige invloed van sommige graansoorten op de lijder aan coeliakie, PhD thesis (in Dutch). Utrecht, the Netherlands: University of Utrecht.
- ^ Fasano A (2009). "Celiac Disease Insights: Clues to Solving Autoimmunity". Scientific American (August): 49–57.
- ^ Anderson CM, French JM, Sammons HG, Frazer AC, Gerrard JW, Smellie JM (1952). "Coeliac disease; gastrointestinal studies and the effect of dietary wheat flour". Lancet. 1 (17): 836–842. doi:10.1016/S0140-6736(52)90795-2. PMID 14918439.
- ^ Paulley JW (1954). "Observation on the aetiology of idiopathic steatorrhoea; jejunal and lymph-node biopsies". Br Med J. 2 (4900): 1318–1321. doi:10.1136/bmj.2.4900.1318. PMC 2080246. PMID 13209109.
- ^ Macdonald WC, Dobbins WO, Rubin CE (1965). "Studies of the familial nature of celiac sprue using biopsy of the small intestine". N Engl J Med. 272 (9): 448–456. doi:10.1056/NEJM196503042720903. PMID 14242522.
- ^ Marks J, Shuster S, Watson AJ (1966). "Small-bowel changes in dermatitis herpetiformis". Lancet. 2 (7476): 1280–1282. doi:10.1016/S0140-6736(66)91692-8. PMID 4163419.
- ^ "Buy Me Some Gluten-Free Peanuts, Cracker Jacks". QSR Magazine. Journalistic. 11 May 2010. Archived from the original on 4 December 2011. Retrieved 30 December 2010.
- ^ Hillson B (9 January 2008). "May as Celiac Awareness Month". Celiac Disease Foundation. Archived from the original on 24 February 2010. Retrieved 1 July 2011.
- ^ "One on-line site sells 1200 wafers weighing a total of 523 g". Eden.co.uk. 16 September 2011. Archived from the original on 16 September 2011. Retrieved 3 September 2013.
- ^ Moriarty KJ, Loft D, Marsh MN, Brooks ST, Gordon D, Garner GV (3 August 1989). "Holy Communion Wafers and Celiac Disease". New England Journal of Medicine. 321 (5): 332. doi:10.1056/NEJM198908033210518. ISSN 0028-4793. PMID 2747779.
- ^ Bentley AC (January 1988). "A Survey of Celiac-Sprue Patients: Effect of Dietary Restrictions on Religious Practices". The Journal of General Psychology. 115 (1): 7–14. doi:10.1080/00221309.1988.9711083. ISSN 0022-1309. PMID 3351491.
- ^ "Statement by the National Conference of Catholic bishops on the use of low gluten hosts at Mass". BCL Newsletter. November 2003. Archived from the original on 16 July 2013.
- ^ "Girl with digestive disease denied Communion". NBC News. Microsoft. Associated Press. 8 December 2004. Archived from the original on 13 November 2013. Retrieved 30 May 2006.
- ^ "The Use of Mustum and Low-Gluten Hosts at Mass". BCL Newsletter. United States Conference of Catholic Bishops. November 2003. Archived from the original on 2 January 2007. Retrieved 7 March 2007.
- ^ McNamara E (15 September 2004). "Liturgy: Gluten-free Hosts". Catholic Online. Archived from the original on 29 September 2007. Retrieved 17 June 2007.
- ^ Millward D (8 July 2017). "Vatican outlaws use of gluten free bread for Holy Communion". The Telegraph. Archived from the original on 4 August 2017. Retrieved 3 August 2017.
- ^ Friedman G (3 April 2017). "Here's the spiel on gluten-free matzah". The Times of Israel. ISSN 0040-7909. Retrieved 25 October 2025.
- ^ "COELIAC DISEASE | meaning in the Cambridge English Dictionary". dictionary.cambridge.org. Retrieved 15 December 2018.
- ^ "Coeliac vs. Celiac". www.glutenfreedublin.com. Archived from the original on 17 December 2018. Retrieved 15 December 2018.
- ^ Kemppainen KM, Lynch KF, Liu E, Lönnrot M, Simell V, Briese T, et al. (May 2017). "Factors That Increase Risk of Celiac Disease Autoimmunity After a Gastrointestinal Infection in Early Life". Clinical Gastroenterology and Hepatology. 15 (5): 694–702.e5. doi:10.1016/j.cgh.2016.10.033. PMC 5576726. PMID 27840181.
- ^ Verdu EF, Caminero A (April 2017). "How infection can incite sensitivity to food". Science. 356 (6333): 29–30. Bibcode:2017Sci...356...29V. doi:10.1126/science.aan1500. PMID 28385972.
- ^ Freeman HJ (November 2013). "Non-dietary forms of treatment for adult celiac disease". World Journal of Gastrointestinal Pharmacology and Therapeutics (Review). 4 (4): 108–112. doi:10.4292/wjgpt.v4.i4.108. PMC 3817285. PMID 24199026.
- ^ Vanga RR, Kelly CP (May 2014). "Novel therapeutic approaches for celiac disease". Discovery Medicine. 17 (95): 285–293. PMID 24882720.
- ^ a b Castillo NE, Theethira TG, Leffler DA (2015). "The present and the future in the diagnosis and management of celiac disease". Gastroenterology Report (Review). 3 (1): 3–11. doi:10.1093/gastro/gou065. PMC 4324867. PMID 25326000.
- ^ Murray JA, Syage JA, Wu TT, Dickason MA, Ramos AG, Van Dyke C, et al. (December 2022). "Latiglutenase Protects the Mucosa and Attenuates Symptom Severity in Patients With Celiac Disease Exposed to a Gluten Challenge". Gastroenterology. 163 (6): 1510–1521.e6. doi:10.1053/j.gastro.2022.07.071. PMC 9707643. PMID 35931103.
- ^ "Entero Therapeutics". enterothera.com. Retrieved 21 May 2024.
- ^ "Innovate Biopharmaceuticals". www.innovatebiopharma.com. Archived from the original on 18 April 2016. Retrieved 17 April 2016.
